
United States Department of Energy
Waste Isolation Pilot Plant
Carlsbad Field Office
Carlsbad, New Mexico

United States Department of Energy
Waste Isolation Pilot Plant
Carlsbad Field Office
Carlsbad, New Mexico
SOTERM-2.0 Expected WIPP Repository Conditions, Chemistry, and Processes
SOTERM-2.1 Ambient Geochemical Conditions
SOTERM-2.2 Repository Conditions
SOTERM-2.2.1 Repository Pressure
SOTERM-2.2.2 Repository Temperature
SOTERM-2.2.3 Water Content and Relative Humidity
SOTERM-2.2.4 Minimum Repository Brine Volume and Variable Brine Volume Implementation
SOTERM-2.3 Repository Chemistry
SOTERM-2.3.2 Brine pH and pH Buffering
SOTERM-2.3.3 Selected MgO Chemistry and Reactions
SOTERM-2.3.4 Iron Chemistry and Corrosion
SOTERM-2.3.5 Chemistry of Lead in the WIPP
SOTERM-2.3.6 Organic Chelating Agents
SOTERM-2.3.7 CPR in WIPP Waste
SOTERM-2.4 Important Post-emplacement Processes
SOTERM-2.4.1 Microbial Effects in the WIPP
SOTERM-2.4.2 Radiolysis Effects in the WIPP
SOTERM-3.0 WIPP-Relevant Actinide Chemistry
SOTERM-3.1 Changes in Actinide Chemistry Information since the CRA-2009 and the CRA-2009 PABC
SOTERM-3.2 Actinide Inventory in the WIPP
SOTERM-3.3.1 Thorium Environmental Chemistry
SOTERM-3.3.2 WIPP-Specific Results since the CRA-2009 and the CRA-2009 PABC
SOTERM-3.4.1 Uranium Environmental Chemistry
SOTERM-3.4.2 WIPP-Specific Results since the CRA-2009 and the CRA-2009 PABC
SOTERM-3.5 Neptunium Chemistry
SOTERM-3.5.1 Neptunium Environmental Chemistry
SOTERM-3.5.2 WIPP-Specific Results since the CRA-2009 and the CRA-2009 PABC
SOTERM-3.6 Plutonium Chemistry
SOTERM-3.6.1 Plutonium Environmental Chemistry
SOTERM-3.6.2 WIPP-Specific Results since the CRA-2009 and the CRA-2009 PABC
SOTERM-3.7 Americium and Curium Chemistry
SOTERM-3.7.1 Americium and Curium Environmental Chemistry
SOTERM-3.7.2 WIPP-Specific Results since the CRA-2009 and the CRA-2009 PABC
SOTERM-3.8 Complexation of Actinides by Organic Chelating Agents
SOTERM-3.8.1 Stability Constants for Organic Complexation with Actinides
SOTERM-3.8.2 WIPP-Specific Data on Organic Complexation Effects Since CRA-2009 and CRA-2009 PABC
SOTERM-3.9.1 Actinide Colloids in the Environment
SOTERM-3.9.2 WIPP-Specific Results since the CRA-2009 and CRA-2009 PABC
SOTERM-4.0 Calculation of the WIPP Actinide Source Term
SOTERM-4.1 Overview of WIPP Approach to Calculate Actinide Solubilities
SOTERM-4.2 Use of Oxidation-State-Invariant Analogs
SOTERM-4.3 Actinide Inventory and Oxidation State Distribution in the WIPP
SOTERM-4.4 Actinide Speciation Reactions Used in EQ3/6
SOTERM-4.4.1 The III Actinides: Pu(III), Am(III), Cm(III)
SOTERM-4.4.2 The IV Actinides: Th(IV), U(IV), Pu(IV), Np(IV)
SOTERM-4.4.3 The V Actinides: Np(V)
SOTERM-4.4.4 The VI Actinides: U(VI)
SOTERM-4.5 Calculations of Actinide Solubility Using the EQ3/6 Computer Code
SOTERM-4.5.1 Pitzer Approach for High-Ionic-Strength Brines
SOTERM-4.5.2 Calculated Actinide Solubilities
SOTERM-4.6 Calculation of Colloidal Contribution to Actinide Solution Concentrations
SOTERM-5.0 Use of the Actinide Source Term in PA
SOTERM-5.1.1 Elements and Isotopes Modeled
SOTERM-5.1.2 Use of Brine End Members
SOTERM-5.1.3 Sampling of Uncertain Parameters
SOTERM-5.1.4 Multiple Brine Volumes
SOTERM-5.1.5 Combining the Transport of Dissolved and Colloidal Species in the Salado
SOTERM-5.2 Construction of the Source Term
SOTERM-5.3 Example Calculation of Actinide Solubility
SOTERM-5.4 Calculated Dissolved, Colloidal, and Total Actinide Solubilities
Table SOTERM- 1. Summary of Current WIPP Chemistry Model Assumptions and Conditions
Table SOTERM- 7. Apparent Stability Constants for Organic Ligands with Selected Metals (NIST 2004)
Table SOTERM-10. Time-dependence of Radionuclide Inventory (Van Soest 2012)
Table SOTERM- 11. Thermodynamic Stability Constants for Key Th Hydrolytic Species
Table SOTERM- 12. Solubility of U(VI) in High-Ionic-Strength Media
Table SOTERM- 17. Oxidation States of the Actinides in the WIPP as Used in the CRA-2014 PA
Table SOTERM- 19. Classification of Four Colloid Types Considered by the WIPP PA
Table SOTERM- 20. Material and Property Names for Colloidal Parameters
% percent
α alpha particle
Aγ Debye-Hückel parameter
ai activity of a chemical species
μ, or µm micrometer, micron
μs microsecond
am amorphous
aq aqueous
ASTP Actinide Source Term Program
atm atmosphere
β (apparent) stability constant, or beta particle
Bq becquerel
BRAGFLO Brine and Gas Flow code
C Celsius; centigrade; concentration
CAPHUM maximum (cap) actinide concentration associated with mobile humic colloids
CAPMIC maximum actinide concentration that could be associated with microbes
CCA Compliance Certification Application
CFR Code of Federal Regulations
Ci Curie
CMC carboxymethylcellulose
CN coordination number
coll colloid
CONCINT actinide concentration associated with mobile actinide intrinsic colloids
CONCMIN actinide concentration associated with mobile mineral fragment colloids
CPR cellulosic, plastic, and rubber materials
CPu maximum concentration of all combined isotopes of Pu
cr crystalline phase
CRA Compliance Recertification Application
DBR direct brine release
D-H Debye-Hückel theory
DNA deoxyribonucleic acid
DOE U.S. Department of Energy
DRZ disturbed rock zone
E0 or Eh potential
EDTA ethylenediaminetetraacetic acid
EDS energy dispersive x-ray spectroscopy
EPA U.S. Environmental Protection Agency
ERDA Energy Research and Development Administration
EQ3/6 software program for geochemical modeling of aqueous systems
eV electron volt
EXAFS Extended X-Ray Absorption Fine Structure
F Fahrenheit
fCO 2 fugacity of carbon dioxide
f(I) Debye-Hückel function
f'(I) derivative of the Debye-Hückel function
FMT Fracture-Matrix Transport
ft foot/feet
g gamma radiation or activity coefficient
g gaseous, or gram, or gravity of Earth
G molecular yield in molecules/100 eV of absorbed ionizing radiation
g/L gram per liter
g/mL gram per milliliter
GBq giga becquerel
GWB Generic Weep Brine
h hours
HEXS high-energy X-ray scattering
hyd hydrated
I ionic strength
ICP-MS inductively coupled plasma-mass spectrometry
ISA isosaccharinic acid
K degree Kelvin or stability constant
kDa kilo Dalton
kg kilogram
Kd dissociation constant
km kilometer
Ks solubility constantKsp solubility product
λij second-order interaction coefficient
L liter
LANL Los Alamos National Laboratory
LANL-CO Los Alamos National Laboratory - Carlsbad Operations
LET Linear Energy Transfer
log logarithm
log10 logarithm base 10
LWB land withdrawal boundary
μijk third-order interaction coefficient
m meter, molal
M mole per liter
m2 square meter
m3 cubic meter
mg milligram
mM millimole per liter
mol mole
molec molecule
MPa megapascal
mV millivolt
n neutron, or number
N degree of polymerization number
nm nanometer
NONLIN Sandia code
NS Adsorption site density (sites/nm2)
NUTS Nuclide Transport System code
OXSTAT oxidation state parameter
P pressure
PA performance assessment
PABC Performance Assessment Baseline Calculation
PANEL Program used in PA
PAVT Performance Assessment Verification Test
pCH+ or pcH Negative logarithm of H+ concentration in moles per liter
pCO2 Partial pressure of carbon dioxide
pH negative logarithm of H+ activity
PHUMCIM Proportionality constant for the actinide concentration associated with mobile humic colloids, in Castile brine
PHUMSIM Proportionality constant for the actinide concentration associated with mobile humic colloids, in Salado brine
pKa negative logarithm of the dissociation constant of an acid
pm picometer
pmH negative logarithm of H+ concentration in molal
ppm parts per million
PROPMIC proportionality constant describing the bioassociation of actinides with mobile microorganisms
ref reference
RH relative humidity
rpm revolutions per minute
s solid or second
SECOTP2D computer program that simulates single or multiple component radionuclide transport in fractures or granular aquifers
SEM scanning electron microscope
Si,b solubility calculated for oxidation state i in brine b
SIT Specific Ion Interaction theory
SNL Sandia National Laboratories
SOTERM Actinide Chemistry Source Term (WIPP)
SPC Salado Primary Constituents
SRB sulfate-reducing bacteria
SUi solubility uncertainty sampled from a distribution unique to each oxidation state i
T temperature
t½ half-life
TDS total dissolved solid
TR-LIF Time-resolved laser induced fluorescence
TRU transuranic
V volt, or vanadium
w with
WIPP Waste Isolation Pilot Plant
WWIS WIPP Waste Information System
XANES X-Ray Absorption Near Edge Structure
XRD X-Ray Diffraction
yr year
zi charge of the specie "i"
Elements and Chemical Compounds
Am Americium
Am(II) Americium in the +2 oxidation state
Am(III) Americium in the +3 oxidation state
Am(IV) Americium in the +4 oxidation state
Am(V) Americium in the +5 oxidation state
Am(VI) Americium in the +6 oxidation state
Am2+ Americium cation - Aqueous form of the americium in the +2 oxidation state that only exists as a transient
Am3+ Americium cation - Aqueous form of the americium in the +3 oxidation state
Am4+ Americium cation - Aqueous form of the americium in the +4 oxidation state
Am(Cl)n (3-n) Americium (III) chloride complex with n = 1 or 2
Am(CO3)n (3-2n) Americium (III) carbonate complex with n=1, 2, 3 or 4
Am OH CO3 Americium (III) carbonato hydroxide
AmO2 + Americium oxo-cation - Aqueous form of the americium in the +5 oxidation state
AmO2 2+ Americium oxo-cation - Aqueous form of the americium in the +6 oxidation state
AmO2OH Americium (V) oxide hydroxide
AmOH2+ Americium (III) hydroxide cation - (1:1) complex
Am(OH)2 + Americium (III) hydroxide cation - (1:2) complex
Am(OH)3 Americium hydroxide
Am(OH)4 - Americium (III) hydroxide anion - (1:4) complex
Am(OH)n (3-n) Americium (III) hydroxide ion - (n:3-n) complex
AmPO4 Americium (III) phosphate
Am(SO4)n (3-2n) Americium (III) sulfate complex with n = 1 or 2
[An]p Concentration of an adsorbed actinide element (mol/particle)
An Actinide
An(III) General actinide in the +3 oxidation state
An(IV) General actinide in the +4 oxidation state
An(V) General actinide in the +5 oxidation state
An(VI) General actinide in the +6 oxidation state
An3+ Aqueous form of the actinide in the +3 oxidation state
An4+ Aqueous form of the actinide in the +4 oxidation state
Ann+ Aqueous form of the actinide in the +n oxidation state
An2(CO3)3 Actinide (III) carbonate - (2:3) complex
An2(CO3)2 2+ Actinide (III) carbonate iaon - (2:2) complex
AnB4O7 + Actinide (III) tetraborate ion - (1:1) complex
AnCl2+ Actinide (III) chloride ion - (1:1) complex
An(CO3)+ Actinide (III) carbonate ion - (1:1) complex
An(CO3)2 - Actinide (III) carbonate ion - (1:2) complex
An(CO3)3 3- Actinide (III) carbonate ion - (1:3) complex
AnCO3OH Actinide (III) carbonate hydroxide
AnL (n+m) Complex of an actinide with a charge n and an organic ligand L with a charge m
An(V)O2 + or AnO2 + Aqueous form of the actinide in the +5 oxidation state
An(VI)O2 2+ or AnO2 2+ Aqueous form of the actinide in the +6 oxidation state
AnOH2+ Actinide (III) hydroxide cation - (1:1) complex
An(OH)3 Hydroxide of the actinide (III)
AnPO4 Actinide (III) phosphate
AnSO4 + Actinide (III) sulfate ion - (1:1) complex
B3O3(OH)4 - Hydroxy polynuclear form of boric acid
B4O7 2- Tetraborate anion
B(OH)x 3-x Hydroxyborate ions
Br- Bromide anion
[C] Concentration of species C in solution
[Cθ] Concentration of a chosen standard state
C Carbon or concentration
C6H10O5 Cellulose
CH4 Methane
CH3CO2 - Acetate anion
(CH2CO2)2C(OH)(CO2)3- Citrate anion
(CH2CO2)2N(CH2)2N(CH2CO2)2 4- Ethylenediaminetetraacetate (EDTA) anion
C2O4 2- Oxalate anion
Ca Calcium
Ca2+ Calcium cation
CaCl2 Calcium chloride
CaCO3 Calcium carbonate
CaMg(CO3)2 Dolomite, calcium magnesium carbonate
Ca[M(OH)3]2+ Calcium metal (III) hydroxide cation - (1:1:3) complex
Ca2[M(OH)4]3+ Calcium metal (III) hydroxide cation - (2:1:4) complex
Ca3[M(OH)6]3+ Calcium metal (III) hydroxide cation - (3:1:6) complex
Cap[Cm(OH)n]3+2p-n Calcium curium (III) hydroxide ion - (p:n:3+2p-n) complex
Ca4[Pu(OH)8]4+ Calcium plutonium (IV) hydroxide cation complex
CaSO4 Anhydrite, calcium sulfate
CaSO4 ×2H2O Gypsum, hydrated calcium sulfate
Ca4[Th(OH)8]4+ Calcium thorium (IV) hydroxide cation complex
Cl Chlorine
Cl- Chloride ion
Cl2 Chlorine
Cl2 - Chlorine free radical
Cl3 - Chlorine anion
ClBr- Chloride bromide radical
ClO- Hypochlorite anion
ClO2 - Chlorite anion
ClO3 - Chlorate anion
ClO4 - Perchlorate anion
Cm Curium
Cm(III) Curium in the +3 oxidation state
Cm(IV) Curium in the +4 oxidation state
Cm3+ Curium cation - Aqueous form of the curium at the +3 oxidation state
Cmm(OH)3m Curium hydroxide polymer
Cm(OH)3 Curium hydroxide
Cm(OH)4 - Curium (III) hydroxide anion - (1:4) complex
CO2 Carbon dioxide
CO3 2- Carbonate anion
Cr Chromium
Cs Cesium
F- Fluoride
Fe Iron
Fe(0), Fe0 Zero-valent iron, metallic iron
FeCO 3 Iron (II) carbonate, ferrous carbonate
Fe2(OH)3Cl Iron -hibbingite, ferrous chloride trihydroxide
Fe 3 O 4 Magnetite, iron (II,III) oxide
Fe2+ Aqueous form of the iron in the +2 oxidation state, ferrous anion
Fe3+ Aqueous form of the iron in the +3 oxidation state, ferric anion
Fe(II) Iron in the +2 oxidation state
Fe(II)(OH)2 Ferrous hydroxide
Fe(III) Iron in the +3 oxidation state
Fe(III)2Fe(II)4(OH)12CO3•2H2O Green rust
Fe(OH) 3 Ferric hydroxide
Fe(OH) 2 ×(x-2)H 2 O Hydrated ferrous hydroxide
FeOOH Goethite, iron oxide hydroxide
FeS Iron (II) sulfideH Hydrogen
H+ Hydrogen cation
H2 Hydrogen
HPO4 2- Hydrogenphosphate anion
HCO3 - Bicarbonate anion, hydrogen carbonate anion
H2O Water
H2O2 Hydrogen peroxide
HOBr Hypobromous acid
HOCl Hypochlorous acid
H2PO4 - Dihydrogen phosphate anion
H 2 S Hydrogen sulfide
K Potassium
K+ Potassium cation
KCl Potassium chloride
K2MgCa2(SO4)4 ×2H2O Polyhalite
KNpO2CO3 ×2H2O Hydrated potassium neptunium (V) carbonate - (1:1:1) complex
K3NpO2(CO3)2 ×0.5H2O Hydrated potassium neptunium (V) carbonate - (3:1:2) complex
KOH Potassium hydroxide
K2SO4 Potassium sulfate
K2U2O7 Potassium diuranate
Li+ Lithium ion
M(III) Metal in the +3 oxidation state
Mg Magnesium
Mg2+ Magnesium cation
MgCl2 Magnesium chloride
Mg3(OH)5Cl·4H2O Magnesium chloride hydroxide hydrate
MgCO3 Magnesite, magnesium carbonate
Mg5(CO3)4(OH)2 ×4H2O Hydromagnesite
Mg2(OH)3Cl×4H2O Magnesium chloride hydroxide hydrate, magnesium oxychloride
MgO Periclase, magnesium oxide
Mg(OH)2 Brucite, magnesium hydroxide
Mn Manganese
N2 Nitrogen
Na Sodium
Na+ Sodium cation
NaBr Sodium bromide
NaCl Sodium chloride
NaClO4 Sodium perchlorate
NaOH Sodium hydroxide
Na2SO4 Sodium sulfate
Na2S2O4 Sodium hydrosulfite
NaAm(CO3)2 Sodium americium (III) carbonate
NaCl Halite, sodium chloride
NaHCO3 Sodium bicarbonate
NaNpO2CO3 ×3.5H2O Hydrated sodium neptunium (V) carbonate - (1:1:1) complex
Na3NpO2(CO3)2 Sodium neptunium (V) carbonate - (3:1:2) complex
NaOH Sodium hydroxide
Na2U2O7 ×xH2O Sodium diuranate hydrate
Nd Neodymium
Nd(III) Neodymium in the +3 oxidation state
Nd(OH)3 Neodymium (III) hydroxide
Ni Nickel
Ni2+ Nickel (II) cation
NO3 - Nitrate anion
Np Neptunium
Np(IV) Neptunium in the +4 oxidation state
Np(V) Neptunium in the +5 oxidation state
Np(VI) Neptunium in the +6 oxidation state
Np4+ Neptunium cation - Aqueous form of the neptunium at the +4 oxidation state
NpO2 Neptunium (IV) oxide
NpO2 + or Np(V)O2 + Neptunyl cation - Aqueous form of the neptunium at the +5 oxidation state
NpO2 2+ or Np(VI)O2 2+ Neptunyl cation - Aqueous form of the neptunium at the +6 oxidation state
NpO5 3- Neptunyl anion - Aqueous form of the neptunium at the +7 oxidation stateNpO2CO3 - Neptunium (V) carbonate ion - (1:1) complex
NpO2(CO3)2 3- Neptunium (V) carbonate ion - (1:2) complex
NpO2(CO3)3 5- Neptunium (V) carbonate ion - (1:3) complex
Np(OH)3 Neptunium (III) hydroxide
Np(OH)4 Neptunium (IV) hydroxide
Np(OH)5 - Neptunium (IV) hydroxide ion - (1:5) complex
NpO2OH Neptunium (V) hydroxide
NpO2(OH)2 Neptunium (VI) hydroxide
NpO2(OH)2 - Neptunium (V) hydroxide ion - (1:2) complexO Oxygen
O2 Molecular oxygen
OBr- Hypobromite anion
OCl- Hypochlorite anion
OH Hydroxide
OH- Hydroxide anion
OH× Hydroxyl radical
Pb Lead
Pb2+ Lead cation - Aqueous form of the lead at the +2 oxidation state
Pb4+ Lead cation - Aqueous form of the lead at the +4 oxidation state
PbCl2 Lead (II) chloride
PbCO3 Lead (II) carbonate
[Pb6O(OH)6]4+ Lead (II) polyoxyhydroxide cation
PbO Lead (II) oxide
PO4 3- Phosphate anion
(PbOH)2CO3 Lead (II) hydroxide carbonate
PbS Lead (II) sulfide
PbSO4 Lead (II) sulfate
Pu Plutonium
Pu(III) Plutonium in the +3 oxidation state
Pu(IV) Plutonium in the +4 oxidation state
Pu(V) Plutonium in the +5 oxidation state
Pu(VI) Plutonium in the +6 oxidation state
Pu(VII) Plutonium in the +7 oxidation state
Pu3+ Plutonium cation - Aqueous form of the plutonium at the +3 oxidation state
Pu4+ Plutonium cation - Aqueous form of the plutonium at the +4 oxidation state
Pu(CO3)+ Plutonium (III) carbonate ion - (1:1) complex
Pu(CO3)2 - Plutonium (III) carbonate ion - (1:2) complex
Pu(CO3)3 3- Plutonium (III) carbonate ion - (1:3) complex
PuF2 2+ Plutonium (IV) fluoride cation
PuO2 Plutonium (IV) dioxide
PuO2+x Oxidized plutonium (IV) dioxide
PuO2CO3 Plutonium (VI) carbonate
PuO2CO3 - Plutonium (V) carbonate ion - (1:1) complex
PuO2(CO3)2 3- Plutonium (V) carbonate ion - (1:2) complex
PuO2(CO3)2 2- Plutonium (VI) carbonate ion - (1:2) complex
PuO2(CO3)3 4- Plutonium (VI) carbonate ion - (1:3) complex
PuO2F+ Plutonium (VI) oxofluoride cation
PuO2 + or Pu(V)O2 + Plutonyl cation - Aqueous form of the plutonium at the +5 oxidation state
PuO2 2+ or Pu(VI)O2 2+ Plutonyl cation - Aqueous form of the plutonium at the +6 oxidation state
PuO2(OH)2 Plutonium (VI) hydroxide
PuO3 ×xH2O Plutonium (VI) trioxide-hydrate
Pu(OH)3 Plutonium (III) hydroxide
Pu(OH)3 + Plutonium (IV) hydroxide cation - (1:3) complex
Pu(OH)4 Plutonium (IV) hydroxide
[Pu(H2O)m]n+ Hydrolysis complex of plutonium
[Pu(O)Pu(O)Pu(O)...]n Plutonium polymer
S2- Sulfide anion
SO4 2- Sulfate anion
Sr Strontium
Th Thorium
Th(IV) Thorium in the +4 oxidation state
Th3+ Thorium cation - Aqueous form of the thorium at the +3 oxidation state
Th4+ Thorium cation - Aqueous form of the thorium at the +4 oxidation state
Th(CO3)5 6- Thorium (IV) pentacarbonyl ion complex
ThISA2 2+ Thorium (IV) isosaccharinic acid ion - (1:2) complex
ThO2 Thorium dioxide
Th(OH)3+ Thorium (IV) hydroxide ion - (1:1) complex
Th(OH)2 2+ Thorium (IV) hydroxide ion - (1:2) complex
Th(OH)3 + Thorium (IV) hydroxide ion - (1:3) complex
Th4(OH)12 4+ Thorium (IV) hydroxide ion - (4:12) complex
Th6(OH)15 9+ Thorium (IV) hydroxide ion - (6:9) complex
Th(OH)4 Thorium hydroxide
Th(OH)(CO3)4 5- Thorium (IV) hydroxide carbonate ion - (1:1:4) complex
Th(OH)2(CO3)2 2- Thorium (IV) hydroxide carbonate ion - (1:2:2) complex
Th(OH)3CO3 - Thorium (IV) hydroxide carbonate ion - (1:3:1) complex
Th(OH)2SO4 Thorium (IV) hydroxide sulfate ion - (1:2:1) complex
Th(OH)4ISA2 2- Thorium (IV) hydroxide isosaccharinic acid ion - (1:4:2) complex
Th(SO4)3 2- Thorium (IV) sulfate ion - (1:3) complex
Th(SO4)2 Thorium (IV) sulfate
Th(SO4)2 ×K2SO4 ×4H2O, Th(SO4)2 ×2K2SO4 ×2H2O, Th(SO4)2 ×3.5K2SO4 Hydrated potassium thorium (IV) sulfate complex
Th(SO4)2 ×Na2SO4 ×6H2O Hydrated sodium thorium (IV) sulfate complex
U Uranium
U(III) Uranium in the +3 oxidation state
U(IV) Uranium in the +4 oxidation state
U(V) Uranium in the +5 oxidation state
U(VI) Uranium in the +6 oxidation state
U3+ Uranium cation - Aqueous form of the uranium at the +3 oxidation state
U4+ Uranium cation - Aqueous form of the uranium at the +4 oxidation state
UO2 Uraninite, uranium (IV) dioxide
UO2 2+ or U(VI)O2 2+ Uranyl cation - Aqueous form of the uranium at the +6 oxidation state
UO2CO3 Rutherfordine, uranium (VI) carbonate
UO2(CO3)2 2- Uranium (VI) carbonate ion - (1:2) complex
UO2(CO3)3 4- Uranium (VI) carbonate ion - (1:3) complex or triscarbonato complex
(UO2)3(CO3)6 6- Uranium (VI) carbonate ion - (3:6) complex
(UO2)2(CO3)(OH)3 - Uranium (VI) carbonate hydroxide ion - (2:1:3) complex
(UO2)11(CO3)6(OH)12 2- Uranium (VI) carbonate hydroxide ion - (11:6:12) complex
UO2(OH)3 - Uranium (VI) hydroxide ion - (1:3) complex
UO2(OH)4 2- Uranium (VI) hydroxide ion - (1:4) complex
U(OH)4 Uranium (IV) hydroxide
UO2.xH2O Hydrous uranium (IV) dioxide
(UO2)(OH)2 ×xH2O or UO3 ×xH2O Schoepite, hydrated uranium trioxide
V Vanadium
ZrO2 Zirconium dioxide
Actinide release from the WIPP is a critical performance measure for the WIPP as a transuranic (TRU) waste repository. There are a number of potential pathways for actinide release considered by the WIPP PA; these are discussed in detail in Appendix PA-2014. Quantifying the impact of these releases contributes directly to assessing compliance with 40 CFR Part 191 (U.S. EPA 1993).
In the undisturbed scenario for PA, actinide releases up the shafts or laterally through the marker beds are insignificant in all realizations and have no impact on compliance (Appendix PA-2014, Section 7 ). The self-sealing of the salt and the reducing anoxic environment in the repository provide the primary mechanisms for geologic isolation of the TRU waste in the undisturbed scenario. For the disturbed scenarios, actinide releases can occur as a result of inadvertent human intrusions (i.e., boreholes drilled into or through the repository). For example, direct brine release (DBR) to the accessible environment may occur during a drilling intrusion, or actinides may be transported up a borehole to the Culebra Dolomite Member of the Rustler Formation and then move laterally through the Culebra to the Land Withdrawal Boundary (LWB). The potential for human intrusions makes it important to assess the range of possible repository conditions and actinide concentrations associated with the disturbed scenarios.
This appendix focuses on the actinide source term used to calculate actinide release from the WIPP for DBR and transport through the Salado Formation and Culebra. This actinide source term is the sum of the soluble and colloidal species in brine. Direct release of actinide particulates to the surface resulting from cuttings, cavings, and spallings is not considered part of the actinide source term because these particulate releases do not depend on the mobilized actinide concentrations in brine.
The relative importance of radioelements (Camphouse et al. 2013) that significantly contribute to the actinide source term, and consequently impact the long-term performance of the WIPP, which is unchanged since the CRA-2009 Performance Assessment Baseline Calculation (PABC) (Clayton et al. 2010), is:
Pu » Am >> U > Th >> Np, Cm, and fission products (SOTERM.1)
The TRU components for this list of radionuclides are the alpha (α)-emitting isotopes of plutonium (Pu), americium (Am), neptunium (Np), and curium (Cm) with half-lives greater than 20 years. These TRU actinides make up the waste unit factor used to calculate the normalized release from the WIPP in U.S. Environmental Protection Agency (EPA) units, as required by 40 CFR Part 191. In SOTERM, the chemistry of thorium (Th) and uranium (U) is also discussed, since these actinides are present in the WIPP waste and their chemistry is analogous to the TRU components.
This appendix has the following overall organization:
- An overview of key near-field conditions and biogeochemical processes is presented in Section SOTERM-2.0.
- An updated literature review and summary of WIPP-relevant results for the key actinides is given in Section SOTERM-3.0.
- A summary of the WIPP actinide PA approach and assumptions, along with the calculated actinide solution concentrations, is provided in Section SOTERM-4.0.
- The PA implementation of the dissolved and colloidal components of the source term is described in Section SOTERM-5.0.
Each of these sections identifies important changes and/or new information since the CRA-2009 (U.S. DOE 2009) and the CRA-2009 PABC (Clayton et al. 2010).
The pre-emplacement and post-emplacement near-field processes and conditions that could affect actinide concentrations in the WIPP are discussed in this section. An up-front summary of the current WIPP chemistry model assumptions and conditions is given in Table SOTERM-1. An up-front summary of the assumptions/role of the engineered barrier and key WIPP-relevant processes is given in Table SOTERM-2. The anticipated inventory of key waste, packaging and emplacement materials in the WIPP is summarized in Table SOTERM-3. All of these are discussed in more detail in the following sections. Emphasis in the detailed description is placed on how these processes and conditions in the repository could affect the concentrations of dissolved and colloi dal actinide species in brine.
Overall, there are relatively few changes in the WIPP repository conditions, chemistry, and processes since the CRA-2009 and the CRA-2009 PABC. New data that support the current WIPP position in some areas were obtained. A preview of these data is given below.
Changes in WIPP repository conditions, chemistry and processes since the CRA 2009 and CRA-2009 PABC:
1) New inventory data, based on the 2012 annual inventory (Van Soest 2012) exist on the amounts of lead, iron and the cellulosic, plastic and rubber (CPR) material in the WIPP. This is summarized in Table SOTERM-3.
2) The minimum brine volume for DBR, which is unchanged at 17,400 m3, is the basis of a variable brine volume PA implementation (Section SOTERM-2.2.4; Appendix PA-2014, Section 1.1.9 ).
3) Brine chemistry and actinide solubilities are now being calculated using EQ3/6 rather than the Fracture Matrix Transport (FMT) program, although the database is essentially the same. This is discussed in Section SOTERM-2.3.1.
4) Modeling and experimental studies to further evaluate the transitional brine chemistry between Generic Weep Brine (GWB) and Energy Research and Development Administration Well-6 (ERDA-6) brines were completed and are described in Section SOTERM-2.3.1. These data support past and ongoing WIPP specific research, but do not impact the WIPP PA.
5) The potential concentration of organic chelating agents has been updated based on new inventory data (Van Soest 2012; Brush and Domski 2013b). These new concentrations are discussed in section SOTERM-2.3.6.
6) Gas generation rates due to corrosion were recalculated based on the new WIPP relevant corrosion rates (Roselle 2013; Section SOTERM-2.3.4; Appendix PA-2014, Section 1.1.4 ).
7) A Significant amount of new data was obtained on the WIPP microbial ecology (Section SOTERM-2.4.1). This new information is centered on indigenous microorganisms in salt from the WIPP and those present in briny groundwaters in the area of the WIPP. Some progress was also made on the aerobic biodegradation of organic chelating agents and the bioassociation of WIPP specific isolates. Although this has provided more insight to the nature of indigenous halophilic microorganisms, we do not have a complete understanding of this microbial ecology and these results have not led to a change in the WIPP microbial model.
Table SOTERM- 1. Summary of Current WIPP Chemistry Model Assumptions and Conditions
|
Repository Condition or Parameter |
CRA-2014 PA Assumptions |
SOTERM-2014 Section |
|
Ambient Geochemistry |
Predominantly halite of the Salado Formation, with anhydrite interbeds and inclusions. |
2.1 |
|
Temperature |
Ambient temperature is 28 oC (82 °F). An increase of up to 3 oC (5.4 °F) is possible as a result of the emplacement of TRU waste. |
2.2.2 |
|
Humidity |
~70 percent (%) relative humidity (RH) at the repository temperature. |
2.2.3 |
|
Water Content |
Host rock is groundwater-saturated with inclusions in the salt that range from 0.057% to 3% by mass. Repository is initially unsaturated until a borehole intrusion occurs. Depending on pressure and intrusion scenarios, the first intrusion will occur between 100 and 1000 years (yrs). |
2.2.3 |
|
Pressure |
A maximum pressure in the repository of about 15 megapascals (MPa) (148 atmospheres [atm]), equivalent to the lithostatic stress at the repository level; a hydrostatic pressure of about 8 MPa (79.0 atm) at the bottom of an intrusion borehole at repository depth. |
2.2.1 |
|
Gas Phase |
Initially air/oxic at repository closure, but rapidly transitions to an anoxic atmosphere dominated by hydrogen with smaller amounts of methane and nitrogen. Trace amounts of carbon dioxide, hydrogen sulfide, and other microbial gases may be present. |
2.2.3 2.4.1 |
|
Disturbed Rock Zone (DRZ) |
Upper bound of 12 meters (m) above the repository and 2 m below the repository horizon. |
2.2.5 |
|
Minimum Brine Volume for DBR |
The calculated minimum volume of brine from any source needed for DBR release is 17,400 cubic meters (m3). This volume is the basis of the variable brine volume approach now used in PA. |
2.2.4 |
|
WIPP Brine |
High-ionic-strength brine that varies with pH and reaction with MgO but is bracketed by GWB and ERDA-6 brine formulations used in the WIPP project. |
2.3.1 |
|
pH |
The expected pH is about 9 (ionic-strength-corrected measured pH (pCH+) of 9.5) and controlled by MgO. The borate and carbonate present add to the brine buffer capacity. |
2.3.2 |
Table SOTERM-2. Assumptions/Role of the Engineered Barrier, Emplaced Waste, and Key WIPP Subsurface Processes
|
Barrier or Process |
CRA-2014 Assumptions and Role in PA |
SOTERM-2014 Section |
|
MgO |
Engineered barrier for the WIPP that will sequester carbon dioxide (CO2) and control increases and decreases in pH by the precipitation of brucite, hydromagnesite, and magnesite. |
2.3.3 |
|
Corrosion |
Container steel and metals in WIPP waste will react to remove oxygen and produce hydrogen. |
2.3.4 |
|
Iron and Lead Chemistry |
The chemistry of iron and lead, which are added to the repository, contributes to our overall understanding of the chemistry of actinides in brine, but this chemistry is selectively implemented in PA. |
2.3.4 and 2.3.5 |
|
Organic Chelating Agents |
The four organic chelating agents addressed by PA are acetate, oxalate, citrate and ethylenediaminetetraacetic acid (EDTA). These are assumed to not degrade under the expected WIPP conditions; their solubility is defined by their inventory (except for oxalate, which is solubility limited); these complex actinides and increase their solubility in the source term. |
2.3.6 |
|
CPR |
These materials are introduced to the WIPP as waste, packaging material and emplacement material. Their biodegradation leads to the formation of carbon dioxide that dissolves in brine to form bicarbonate/carbonate species that impact pH and complex actinides. |
2.3.7 |
|
Microbial Effects |
Gas generation, primarily carbon dioxide and hydrogen sulfide, resulting from the biodegradation of CPR materials and creation of reducing conditions, including bioreduction of actinide elements from higher oxidation states. Microbial processes are assumed to occur in all PA realizations. |
2.4.1 |
|
Radiolysis |
Localized oxidizing effects possible near high-activity actinides, but overall radiolytic processes are overwhelmed by the in-room chemistry. |
2.4.2 |
Table SOTERM- 3. Total Projected Waste, Packaging and Cement Material in the WIPP Repository (Van Soest 2012)
|
Material |
Source/Type |
*Amount (kg) |
Total (kg) |
|
Iron-based metals/alloys |
Waste |
1.22 × 107 |
4.91 × 107 |
|
Packaging |
3.69 × 107 |
||
|
Aluminum-based metals/alloys |
Waste |
4.57 × 105 |
4.57 × 105 |
|
Lead |
Packaging |
8.28 × 103 |
8.28 × 103 |
|
Cellulosics |
Waste |
3.66 × 106 |
4.65 × 106 |
|
Packaging |
7.23 × 105 |
||
|
Emplacement |
2.6 × 105 |
||
|
Plastics |
Waste |
5.50 × 106 |
9.51 × 106 |
|
Packaging |
2.77 × 106 |
||
|
Emplacement |
1.25 × 106 |
||
|
Rubber |
Waste |
1.18 × 106 |
1.25 × 106 |
|
Packaging |
7.33 × 104 |
||
|
CPR Total |
Waste |
1.03 × 107 |
1.54 × 107 |
|
Packaging |
3.57 × 106 |
||
|
Emplacement |
1.51 × 106 |
||
|
Cement |
Reacted |
4.22 × 106 |
1.08 × 107 |
|
Combination |
6.55 × 106 |
||
|
MgO |
Emplacement |
N/A |
51,430 tons |
|
Organic Ligands (all from waste) |
Acetate |
9.96 × 103 |
2.41 × 104 |
|
Acetic Acid |
1.41 × 104 |
||
|
Oxalate |
6.50 × 102 |
1.85 × 104 |
|
|
Oxalic Acid |
1.78 × 104 |
||
|
Citrate |
2.55 × 103 |
7.78 × 103 |
|
|
Citric Acid |
5.23 × 103 |
||
|
EDTA |
3.76 × 102 |
3.76 × 102 |
|
|
*Includes remote-handled and contact-handled waste sources when applicable. |
|||
The ambient geochemical conditions are discussed in detail in the Compliance Certification Application (CCA) (U.S. DOE 1996) and the CRA-2004, Chapter 2 and Chapter 6, Section 6.4.3 (U.S. DOE 2004). The Salado, which is the host formation, is predominantly pure halite (NaCl), with interbeds (marker beds) consisting mainly of anhydrite (CaSO4). The nearly pure halite contains accessory evaporite minerals such as anhydrite (CaSO4), gypsum (CaSO4 ×2H2O), polyhalite (K2MgCa2(SO4)4 ×2H2O), magnesite (MgCO3), and clays. Small quantities of intergranular (grain-boundary) brines and intragranular brines (fluid inclusions) are associated with the salt at the repository horizon. These brines are highly concentrated solutions (ionic strength up to 8 moles per liter [M]) of predominantly sodium (Na+), magnesium (Mg2+), potassium (K+), chloride (Cl-), and sulfate (SO4 2−), with smaller amounts of calcium (Ca2+), carbonate (CO3 2−), and borate (B(OH)4 − and/or B4O7 2−). These brines have been in contact with the Salado evaporite minerals since their deposition (estimated to be 250 million years) and are saturated with respect to these minerals.
Underlying the Salado is the Castile Formation, composed of alternating units of interlaminated carbonate, anhydrite, and nearly pure halite. The Castile in the vicinity of the WIPP site is known to contain localized brine reservoirs with sufficient pressure to force brine to the surface if penetrated by a borehole. Castile brines are predominantly saturated NaCl solutions containing Ca2+ and SO4 2-, as well as small concentrations of other elements, and are about eight times more concentrated than seawater. Overlying the Salado in the vicinity of the WIPP site is the Culebra Dolomite Member of the Rustler Formation, a fractured dolomite (CaMg(CO3)2) layer. It is significant because it is expected to be the most transmissive geologic pathway to the accessible environment. Culebra brines are generally more dilute than the Salado and Castile brines, and are predominantly NaCl with K+, Mg2+, Ca2+, SO4 2-, and CO3 2-. More detailed information on the distribution of Culebra brine salinity in the WIPP site and vicinity can be found in Appendix HYDRO-2014.
Repository conditions that could potentially affect actinide solubility are briefly summarized in this section. These include repository pressure, repository temperature, water content and relative humidity, the minimum free volume for actinide release (effective porosity), and the extent of the DRZ.
The preexcavation lithostatic pressure (Stein 2005; Appendix PA-2014, Section 4.2.4 ) in the WIPP at repository depth is about 15 MPa (148 atm). This pressure can be reestablished after repository closure due to salt creep and gas generation, but there are a number of PA vectors that predict pressure may not be fully restored even by the end of the 10,000-yr period of WIPP performance, and final pressures may range from 6 to 15 MPa (in the undisturbed scenario) and from 0.1 to 15 MPa (in the disturbed scenarios) considered in the CRA-2014 PA. In this context, the pressure in the repository after closure cannot significantly exceed the far-field confining stress of about 15 MPa.
DBR can occur when the pressure in the repository at the time of a drilling intrusion exceeds 8 MPa and a sufficient amount of brine has already flowed into the repository (see related discussions in Section SOTERM-2.2.4, Stein (Stein 2005) and Clayton (Clayton 2008)). Eight MPa is the pressure exerted by a column of brine-saturated drilling fluid at the depth of the repository (Stoelzel and O'Brien 1996). For repository pressures less than 8 MPa, no DBRs are assumed to occur because the fluid pressure in the repository cannot eject the drilling fluid from the borehole. There is also no DBR until the brine volume exceeds the minimum brine volume (see Section SOTERM-2.2.4) needed to fill the effective porosity present in the compacted TRU waste.
The range of pressures expected in the WIPP will not likely have an impact on actinide solubility. The maximum pressure possible (~15 MPa) is well below pressures needed to affect the solution chemistry, and is not expected to have a significant effect on actinide solubilities or processes that lead to the association of actinides with colloidal particles. For these reasons, the effect of pressure on actinide solubility is not considered in the WIPP PA.
The ambient pre-emplacement temperature at the WIPP repository horizon is 27 degrees centigrade (ºC) (80 degrees Fahrenheit (ºF)) (Bennett et al. 1996). The emplacement of TRU waste in the WIPP introduces possible exothermic reactions: MgO hydration, MgO carbonation, microbial degradation, aluminum corrosion and cement hydration. The potential contributions of each of these processes were re-evaluated for the CRA-2014 (see Appendix SCR-2014, Section 6.3.4.1.3 ) and leads to a maximum possible temperature increase of up to 39 ºC (12 ºC increase). These elevated temperatures are expected to persist for a short period of time, perhaps a few years or decades. This is also discussed in Sanchez and Trellue (Sanchez and Trellue 1996) and Wang and Brush (Wang and Brush 1996). For the purposes of PA, the temperature of the WIPP underground repository is assumed to be constant with time at 300 Kelvin (K) (27 ºC [80 ºF]) (Appendix PA-2014, Section 4.2.2 ).
Actinide solubilities were calculated in the WIPP PA using thermodynamic and laboratory data measured at 25 ºC [77 ºF]. The expected effect of the slightly elevated temperature in the WIPP on actinide concentrations is relatively small, especially when compared to other uncertainties inherent in the measurement and calculation of the actinide solubilities and colloidal concentrations. For this reason, the very small effect of temperature on actinide solubility was not considered in the WIPP PA calculations.
A key argument for the WIPP as a TRU waste repository is that the self-sealing of the salt will limit the availability and transport of water into and through the repository, and correspondingly minimize the potential release of TRU nuclides from the repository. In all the undisturbed repository scenarios considered by PA, no significant release of actinides from the WIPP is predicted (Appendix PA-2014, Section 7 ). There is, however, groundwater in the WIPP, even in undisturbed scenarios, that is potentially available to interact with the TRU waste. The salt surrounding the waste is groundwater-saturated with both intergranular and intragranular water. The amount of water present as inclusions in the salt is effectively used as an uncertain parameter in PA calculations with a range of 0.057 to 3 weight % based on what was measured in preexcavation salt (Skokan et al. 1987; Powers et al. 1978). In PA (Appendix PA-2014, Section 4.2.4 ) this is done indirectly by sampling a range in the halite porosity for the intact and DRZ salt (0.001 to 0.0519 and 0.0038 to 0.0548 respectively - see Ismail 2007). Available brine can seep into the repository horizon and fill the pore volume of the transuranic (TRU) waste in the excavated areas. The presence of some brine in the WIPP prior to brine saturation leads to an environment that will contain an atmosphere of up to about 70% RH, defined by the vapor pressure of saturated brine at the repository temperature. This water vapor pressure will be present, at least in part, until brine saturation occurs as a result of some human intrusions or brine seepage into the excavated area.
The presence of a humid environment in the WIPP prior to brine saturation may have a transitory effect on actinide solubilities. These transitory/temporary phases are not considered in the WIPP PA because they will be rapidly overwhelmed by the in-room chemistry and higher reactivity of the waste components should brine inundation or saturation occur.
The minimum brine volume is the lowest amount of brine needed for a DBR to occur during an intrusion scenario. Two criteria must be met:
1) Volume-averaged pressure in the vicinity of the repository encounter by drilling must exceed the drilling fluid hydrostatic pressure
2) Brine saturation in the repository must exceed the residual saturation of the waste material
The minimum brine volume is given by the following:
Minimum brine volume = (median sampled residual brine saturation)
x (equivalent repository rooms) (SOTERM.2)
This was most recently recalculated by Clayton (Clayton 2008) to be 17,400 m3. This 17,400 m3 value corresponds to a consolidated void volume of 523.1 m3, 120.3 equivalent rooms in the repository, and a median value for the sampled residual brine saturation of 0.276. These parameters were calculated based on the method recommended by Stein (Stein 2005), except that the drilling fluid hydrostatic pressure (8 MPa) was used rather than the lowest pressure realization at 10,000 years. This change makes the minimum volume calculation more consistent with the DBR conceptual model.
The minimum repository brine volume has two important potential impacts on calculating actinide concentrations in the WIPP. The first is that the predicted inventory of some actinides, when fully dissolved in this brine volume, lead to concentrations that are below their predicted solubility, most importantly Np and Cm. In this context, they are assumed to be fully dissolved in the brine and since their inventory-limited concentration is small, the impact on the calculated actinide release is insignificant. The second impact is on the predicted concentration of key organic and inorganic complexants that coexist with the TRU species in WIPP waste. The maximum concentrations of acetate, citrate, and EDTA (see Section SOTERM-2.3.6) are defined by their fully dissolved concentration in this minimum brine volume.
The DRZ is a zone immediately surrounding the excavated repository that has been altered by the construction of the repository. A more detailed discussion of the DRZ can be found in Appendix PA-2014, Section 4.2.4. In the Brine and Gas Flow (BRAGFLO) code, the Upper DRZ has a height of about 12 m (39 feet [ft]) and the Lower DRZ has a depth of about 2.2 m (7.2 ft). The creation of this DRZ disturbs the anhydrite layers and marker beds and alters the permeability and effective porosity of the rock around the excavated areas, providing enhanced pathways for the flow of gas and brine between the waste-filled rooms and the nearby interbeds.
The DRZ is important to the calculation of dissolved actinide concentrations because it potentially makes the minerals in the interbeds "available" for reaction with the TRU and emplaced waste components. The most important of these minerals is the calcium sulfate (anhydrite) that could function as a source of sulfate for processes in the repository subsequent to brine inundation. Currently, sulfate is assumed to be available from the DRZ into the waste area, which prolongs microbial sulfate reduction processes in the WIPP.
Brine present in the WIPP will react with emplaced TRU waste, waste components, and the engineered barrier material to establish the brine chemistry that will define actinide solubilities and colloid formation. At the repository horizon, the brine composition will be defined by a combination of factors that include the initial composition of the in-flow brine; reactions that control pH; and the extent to which this brine is altered by equilibration with the waste components, emplaced container materials, and the waste-derived organic chelating agents that can dissolve in the brine. An overview of this repository chemistry is given in this section.
Salado brine will enter the repository after closure, and can be supplemented by Castile brine in some human intrusion scenarios. It is also possible that groundwater from the Rustler and Dewey Lake Formation could flow down the borehole into the repository, mix with the waste, and then be forced back up a borehole. The majority of WIPP-specific solubility studies since the CRA-2004 were performed using brines that bracket the expected range in brine composition. Including brine mixing in PA has been considered and rejected because using the end member brines (i.e., GWB or ERDA-6 brines) brackets the median values and uncertainties for the solubility calculations.
In addition to using these end-member brines in PA, other simplifying assumptions are also made:
1. Any brine present in the repository is well mixed with waste.
2. Equilibria with halite and anhydrite, the most abundant Salado minerals at or near the stratigraphic horizon of the repository, are rapidly established.
3. Oxidation-reduction (redox) equilibria with waste materials are not assumed.
4. Brine compositions attained after equilibration of GWB or ERDA-6 with the MgO engineered barrier exist for the entire 10,000-year regulatory period.
The composition of brine in and around the WIPP site prior to waste emplacement was established by sampling the groundwater and intergranular inclusions in the Salado and Castile (Popielak et al. 1983; Snider 2003a). A number of synthetic brines that simulate these compositions were developed and have been used for WIPP laboratory studies (Lucchini et al. 2013c, Table 1). Currently, the two simulated brines that best represent these repository-relevant, end-member brines are: (1) GWB, which simulates intergranular (grain-boundary) brines from the Salado at or near the stratigraphic horizon of the repository (Snider 2003a); and (2) ERDA-6, which simulates brine from the ERDA-6 well, typical of fluids in Castile brine reservoirs (Popielak et al. 1983).
The reaction of GWB and ERDA-6 brines with MgO (brucite), halite, anyhydrite, and hydromagnesite leads to some potentially significant changes in the composition of the brine (Table SOTERM-4). These brines were reacted using EQ3/6 version 8.0a and database DATAA0.FMT.R2 (Brush and Domski 2013a). The most important of these changes for GWB brine is the lowering of the magnesium concentration from 1.02 to 0.330 M, a decrease in calcium concentration from 14 to 11.1 mM, and a pH of 8.82. For ERDA-6, there is a significant increase in the magnesium concentration from 19 to 136 millimoles per liter (mM), a decrease in total inorganic carbon from 16 to 0.455 mM, and an increase of the pH to 8.99 from 6.17. The pH associated with these MgO-reacted brines established the range of expected pH values in the WIPP for the calculation of actinide solubilities, and the composition of these reacted brines were used in PA to calculate actinide solubility in brine (Brush and Domski 2013a).
There are new data that validate the bracketing approach being used in the WIPP PA since the CRA-2009 submittal. Modeling (Brush et al. 2011) and experimental (Lucchini et al. 2013c) studies were conducted to investigate the pH dependency and the long-term stability of WIPP-specific brines. This was done to assess the validity of using the GWB and ERDA-6 formulations as bracketing brines in the solubility studies and establishes a broad-pH range comparison between modeling and experimental results.
The long-term stability of the unused GWB and ERDA-6 simulated brines (95% composition), used in actinide solubility studies showed no pattern of instability or precipitation. These results confirmed that the 95% formulations of the GWB and ERDA-6 brine were stable for up to six years and that the methods used for storage were appropriate and adequate during this time. The concentration of the brine components in the long-term uranium, neodymium and plutonium solubility and redox studies were also measured to determine their stability under the broader range of pH and experimental conditions used (pCH+ of 6-12, presence of actinides/analogs, presence of carbonate, presence of iron). Under this broader set of interactions, the only changes noted were the precipitation of borate and magnesium salts in the higher-pH ERDA-6 experiments (pCH+ > 10).
The effect of pCH+ on WIPP simulated brines was also investigated and modeled. GWB (100% formulation) was stepwise titrated up to pCH+ ~ 13, and the brine component concentrations were determined after 3-week equilibrations. These experimental results were compared with the predicted composition of the brine using the current WIPP brine model (Figures SOTERM-1 and SOTERM-2). Overall, there was good agreement between the experimental and the modeling results at pCH+ ≤ 10.5 (which includes the pCH+ predicted for the expected conditions in the WIPP). The one exception to this is the decrease in tetraborate concentrations to ~2×10-3M between pCH+ of 10 and 10.5 (Figure SOTERM-1) since there are currently no Pitzer parameters for tetraborate in the WIPP model.
At pCH+ ≥10.5, there were a number of explainable discrepancies noted between the experimental and modeling results for Mg2+, Ca2+ and tetraborate (Figures SOTERM-1 and SOTERM-2). Specifically, calcium precipitation was only observed experimentally at pCH+ > 10.5; magnesium remains in solution above pCH+ 10.5 in the experiments performed and does not precipitate to the extent predicted by the model; and the tetraborate concentration goes through a minimum at pCH+ 9.75 that is also not captured in the modeling results. These results are explained by precipitation of calcium carbonate, as it was observed in the experiments of Kerber Schütz et al. (Kerber Schütz et al. 2011), and the resolubilization of magnesium due to a change in the speciation of tetraborate at high pCH+ (Schweitzer and Pesterfield 2010).
Overall, the modeling and experimental brine chemistry studies established a better understanding of the actinide-relevant brine chemistry over a wider range of experimental conditions than previously studied. GWB and ERDA-6 were confirmed as good "bracketing" brines for WIPP-relevant studies, as GWB brine transitions into ERDA-6 at pCH+ ~10.5. Relatively good agreement was found between the long-term experiments (using 95% formulation brines) and the titration experiments (using the 100% formulation GWB). All of these results effectively increase the robustness of the current WIPP model and provide a better foundation for future and ongoing WIPP-relevant actinide solubility studies.
Table SOTERM- 4. Composition of GWB and ERDA-6 Brine Before and After Reaction with Anhydrite, Brucite and Hydromagnesite. The reacted brine compositions were used to calculate actinide solubilities for the CRA-2014 PA.
|
GWB
|
GWB |
ERDA-6 Brine Compositiond |
ERDA-6 |
|
|
B(OH)x 3-x (see footnote e) |
158 mM |
186 mM |
63 mM |
62.3 mM |
|
Na+ |
3.53 M |
4.77 M |
4.87 M |
5.30 M |
|
Mg2+ |
1.02 M |
0.330 M |
19 mM |
136 mM |
|
K+ |
0.467 M |
0.550 M |
97 mM |
96.0 mM |
|
Ca2+ |
14 mM |
11.1 mM |
12 mM |
11.6 mM |
|
SO4 2- |
177 mM |
216 mM |
170 mM |
182 mM |
|
Cl- |
5.86 M |
5.36 M |
4.8 M |
5.24 M |
|
Br- |
26.6 mM |
31.3 mM |
11 mM |
10.9 mM |
|
Total Inorganic C (as HCO3 -) |
Not reported |
0.379 mM |
16 mM |
0.455 mM |
|
pH |
Not reported |
8.82 |
6.17 |
8.99 |
|
Ionic Strength (M) |
7.44 |
6.44 |
5.32 |
5.99 |
|
a - ions listed represent the total of all species with this ion. b - From Snider 2003a c - From Brush, Domski and Xiong 2011 d - From Popielak et al. 1983 e - Boron species will be present in brine as boric acid, hydroxyl polynuclear forms (B3O3(OH)4 -, and/or borate forms (e.g., B4O7 -) |
||||
Figure SOTERM- 1. Comparison of Experimentally-measured (Lucchini et al. 2013c) and Model-predicted (Brush et al. 2011) Concentrations of Tetraborate and Mg2+in GWB 100% Saturated Brine as a Function of pCH+.
Figure SOTERM- 2. Comparison of Experimentally-measured (Lucchini et al. 2013c) and Model-predicted (Brush et al. 2011) Concentrations of Na+, K+, Ca2+ and Li+ in GWB 100% Saturated Brine as a Function of pCH+. Li+ was not considered in the numerical simulation.
The brine pH is a very critical parameter in defining the solubility of actinides under conditions where brine-mediated releases (DBR and transport through the Culebra) would be important in the WIPP. There are a number of highly-coupled processes that can influence the pH when the emplaced TRU waste is inundated with brine. The most important of these are the potential buffering capacity of the brine coming into the WIPP, the reactions of this brine with emplaced waste components (most notably reduced metals and organics), and microbial processes. The reactions of the emplaced MgO barrier material are expected to sufficiently control and define the pH when the repository is saturated with brine. Although there have been modeling and experimental studies to investigate the pH of WIPP-specific brines, there is no significant change in the key arguments for brine pH and pH buffering since the CRA-2009.
The range of brine composition that is likely to be present in the WIPP repository was discussed in Section SOTERM-2.3.1 (see also Table SOTERM-4). These brines have an intrinsic buffering capacity that is highest at pH 8.5-9. ERDA-6 brine, although it has an ambient pH of 6.2, contains a number of constituents that, in the pH range of 8-10, add buffer capacity to the reacted brine: carbonate/bicarbonate (16 mM), borate (63 mM), and divalent cations that tend to react with hydroxide or carbonate to influence pH (Ca2+ at 12 mM, and Mg2+ at 19 mM). The pKa for boric acid and dissolved carbonate/bicarbonate species are 9.0 and 9.67, respectively, which explains the tendency of this brine to maintain the pH in the range of 8-10. Operationally, the simulated ERDA-6 brines prepared in the laboratory have relatively high buffering capacity, and significant changes in brine concentrations and pH are not routinely observed once the pH is experimentally defined (Lucchini et al. 2013c). An operational pH range for ERDA-6 has been defined as having an upper limit of pH ~10, which is the pH at which a cloud point (indicating magnesium (Mg) precipitation) is observed. The pre-excavation ambient ERDA-6-like brine will naturally add to the buffering capacity of the WIPP brine due to its acid-base components and will establish a relatively high buffer capacity at the mildly alkaline conditions expected in the WIPP.
The expected pH in the WIPP in the event of brine saturation, however, will be defined by the reaction of the Castile ERDA-6-like brine with the waste components and barrier material. This was re-evaluated as part of the documentation for the CRA-2014 PA (Brush et al. 2011; Table SOTERM-4; Brush and Domski 2013a; Lucchini et al. 2013c). The hydration and carbonation reactions of MgO are discussed extensively in Appendix MgO-2009. In PA, the following two reactions combine to define fCO2 and the pH:
5Mg(OH)2 + 4CO2(aq or g) D Mg5(CO3)4(OH)2 ×4H2O (SOTERM.3)
Mg(OH)2 D Mg2+ + 2OH- (SOTERM.4)
Calcite formation (see reaction SOTERM.5) may also occur (see Figure SOTERM-2). This reaction is not considered in PA and remains a conservatism in the current PA model.
Mg(OH)2 + Ca2+ + CO2(aq or g) D CaCO3 + Mg2+ + H2O(aq or g) (SOTERM.5)
In PA, all vectors assume microbial activity consume organic material to produce CO2 (see more detailed discussion in Section SOTERM-2.4.1.1). Carbon dioxide production, if not for its sequestration by MgO, would over time acidify any brine present in the repository and increase the solubility of the actinides relative to that predicted for near-neutral and mildly basic conditions. Current repository assumptions lead to a calculated fCO2 of 3.14 x 10-6 atm (10-5.50 atm) in both GWB and ERDA-6 and a predicted pH of 8.82 and 8.99 respectively. These fCO2 and pH values were used in the actinide speciation and solubility calculations for all CRA-2014 PA vectors.
There are no new WIPP-specific results to report that explicitly address the MgO buffering of the WIPP brine since the CRA-2009. The brine titration experiments and calculations that were performed were described in section 2.3.1. These data are consistent with experimental results published previously by the German program (Altmaier et al. 2003) that were discussed more extensively in Appendix SOTERM-2009, section 2.3.2. All of these data suggest that MgO controls the pH to a pH = 9 ± 1. In this context, it is predicted that brine pH will remain between 8 and 10 under the range of expected conditions in the WIPP.
MgO is the bulk, granular material emplaced in the WIPP as an engineered barrier. The MgO currently being placed in the WIPP contains 96 ± 2 mol % reactive constituents (i.e., periclase and lime) (Deng et al. 2006; Reyes 2008). The amount of MgO emplaced in the WIPP is currently calculated based on the estimated CPR content with an excess factor of 1.2, and it is estimated that in excess of 50,000 metric tons will be emplaced in the WIPP by the time of repository closure.
The chemistry of MgO is critical to the overall performance of the WIPP and is discussed in detail in Appendix MgO-2009. The most recent data are described in Xiong and Lord (Xiong and Lord 2008). The MgO, as the engineered barrier in the WIPP repository design, has two important functions that directly support the PA calculation of actinide concentrations in brine:
1. Sequester the excess CO2 produced by the microbial consumption of CPR material and establish/maintain a low fCO2 in the repository (see reaction SOTERM.3). This is currently estimated to be 10-5.5 atm for GWB and ERDA-6 brine.
2. Establish and buffer the brine pH by maintaining a magnesium solution concentration that reacts with hydroxide (see reaction SOTERM.4) to buffer the pH at about 9. This was part of the pH discussion in Section SOTERM-2.3.2. This buffering removes uncertainty from the actinide concentration calculations.
Initially, MgO will undergo hydration to generate brucite (Mg(OH)2). In time, brucite will react further to form magnesium chloride hydroxide hydrate (e.g., Mg3(OH)5Cl·4H2O) in Salado brine (Appendix MgO-2014, section MgO-4.1 ). These phases combine to control the concentration of magnesium in high-magnesium brine (for example, GWB). The existence of magnesium as an aqueous (aq) cation in equilibrium with excess magnesium minerals helps to establish the solution pH.
For the reaction of MgO with GWB brine, PA uses a magnesium concentration of ~0.33 M (Table SOTERM-4), which is supported by experimental results showing a magnesium concentration ~0.3 M (Lucchini et al. 2013c). This reaction was also investigated by Altmaier et al. (Altmaier et al. 2003) and Harvie, Møller, and Weare (Harvie, Møller, and Weare 1984). Snider also noted that the rate of MgO hydration is most likely linked to mineral phase changes between hydrated magnesium oxychloride and brucite (Snider 2003b). The existence of the hydrated magnesium oxychloride phase was inferred from scanning electron microscope (SEM) images, coupled with an energy dispersive x-ray spectroscopy system (EDS), to identify Mg-Cl phases. The Altmaier and Harvie studies showed that the hydration reaction was a solid-phase transformation between brucite and hydrated magnesium oxychloride that depends not on magnesium concentration, but on chloride concentration, with an invariant point predicted at 1.8 m MgCl concentration and a -log mH+ = 8.95.
The most important role of the MgO engineered barrier is to sequester carbon dioxide to maintain a low fCO2 in the repository. Microbial consumption of CPR materials could produce significant quantities of CO2. Under these conditions, brucite and magnesium chloride hydroxide hydrate will react with the CO2 generated. Both laboratory and modeling studies predict that the following carbonation reaction will buffer fCO 2 at a value of 10-5.50 atm in both GWB and ERDA-6:
5Mg(OH)2 + 4CO2(aq or g) D Mg5(CO3)4(OH)2 ×4H2O (SOTERM.6)
This reaction effectively removes excess CO2 from the repository and bicarbonate/carbonate from the brine. The initial product of MgO carbonation reaction is Mg5(CO3)4(OH)2·4H2O. This is converted into MgCO3, which is the expected stable mineral form of magnesium carbonate in the WIPP, according to Reaction SOTERM.7:
Mg5(CO3)4(OH)2 × 4H2O + CO2(aq or g) + 10 H2O D 5 MgCO3∙3H2O (SOTERM.7)
Reaction SOTERM.6 is slow and it is estimated that hundreds to thousands of years (Appendix MgO-2009; Clayton 2013) are needed for the conversion of hydromagnesite to magnesite. Consumption of CO2 will prevent the brine acidification, and magnesium carbonate precipitation will maintain low carbonate concentration in the WIPP brine to avoid the formation of highly soluble actinide species with carbonate complexes. Although MgO will consume essentially all CO2, residual quantities in equilibrium with magnesite under the WIPP conditions will persist in the aqueous and gaseous phases.
The importance of magnesium chemistry, and correspondingly the chemistry associated with the emplaced MgO on the calculation of actinide concentrations in brine is clear. MgO sequesters CO2 and minimizes the buildup of carbonate in brine. At the expected pH, carbonate forms strong complexes with the An(III), An(IV), and An(VI) oxidation states. An increased carbonate concentration in brine would significantly increase actinide solubilities. Additionally, MgO helps establish the pH in brine. The removal of CO2 prevents a decrease in the pH that could also significantly increase actinide solubility. An additional beneficial effect of MgO is to maintain a solution concentration of Mg2+ that will precipitate as brucite to keep the pH in the 8-10 range. The presence of MgO leads to a more predictable chemistry that lowers the uncertainty when calculating actinide concentrations in the WIPP brine.
The WIPP repository will contain a large quantity of reduced iron due to the use of iron-based containers for much of the emplaced TRU waste. Currently, it is estimated that the WIPP will contain over 49,000 metric tons of iron (Van Soest 2012) when all the waste is emplaced. The presence of this reduced metal will have an important role in the establishment of reducing conditions in the WIPP by removing oxygen. Reduced iron species (aqueous Fe(II) and Fe(0, II)-valent minerals) are important because they will reduce higher-valent actinides in the WIPP, leading to lower actinide solubilities (Section SOTERM-3.6; Reed et al. 2009; Reed et al. 2006). The role of iron in the WIPP PA is unchanged since the CRA-2009, although new data on WIPP-specific corrosion rates were obtained that are now the basis of gas generation rates due to corrosion (Appendix PA-2014, Section1.1.4).
The chemistry of iron will have a pronounced effect on WIPP-relevant actinide chemistry in many ways. The linkages of iron chemistry to the redox chemistry are well established in the literature (Farrell et al. 1999; Fredrickson et al. 2000; Qui et al. 2001; Nakata et al. 2004; Behrends and Van Cappellen 2005). Iron will establish reducing conditions conducive to the overall reduction of higher-valent actinide species and precipitate an iron sulfide phase that removes sulfide from solution. Additionally, iron species could sequester carbon dioxide and compete with actinides for organic and inorganic complexants, although there is no explicit credit taken for this in the WIPP PA.
It is expected that oxic corrosion of steels and aerobic microbial consumption of CPR materials will quickly consume the limited amount of oxygen (O2) trapped within the repository at the time of closure. After O2 is consumed, anoxic corrosion of metals will occur (Brush 1990; Brush 1995; Wang and Brush 1996; Roselle 2013). In all of the vectors for the 2009 PA, the CRA-2009 PABC, the 2004 PA, the CRA-2004 PABC, the CCA PA, and the EPA's CCA 1997 Performance Assessment Verification Test (PAVT), there were significant amounts of uncorroded steels and other Fe-base alloys in the repository throughout the 10,000-yr regulatory period. The WIPP-specific experiments (Telander and Westerman 1993; Telander and Westerman 1997) showed that steels and other Fe-based alloys will corrode by the following reactions:
Fe + (x)H 2 O D Fe(OH) 2 ×(x-2)H 2 O + H 2 ; (SOTERM.8)
3Fe + 4H 2 O D Fe 3 O 4 + 4H 2 ; (SOTERM.9)
Fe + H 2 O + CO 2 D FeCO 3 + H 2 ; and (SOTERM.10)
Fe + H 2 S D FeS + H 2 . (SOTERM.11)
Since the experiments of Telander and Westerman (Telander and Westerman 1993; Telander and Westerman 1997), a new series of steel and lead corrosion experiments has been conducted (Roselle 2009, Roselle 2010, Roselle 2011a, Roselle 2011b, and Roselle 2013). The object of these experiments has been to determine steel and lead corrosion rates under WIPP-relevant conditions. Telander and Westerman (Telander and Westerman 1993; Telander and Westerman 1997) measured H2 generation rates directly and from those measurements were then able to calculate metal corrosion rates. However, the new experiments directly measured metal corrosion rates based on mass loss (Roselle 2013). These new experiments showed that it is possible for other corrosion products (e.g., green rust, hibbingite, etc.) to form (Roselle 2009, Roselle 2010, Roselle 2011a, Roselle 2011b, and Roselle 2013; Nemer et al. 2011). In fact, Roselle (Roselle 2013) states that green rust is the most likely corrosion product in experiments with low atmospheric CO2 concentrations (<350 ppm). At higher concentrations of CO2 (>1500 ppm) iron carbonate was seen as the major corrosion product (forming via SOTERM.10). Assuming an idealized formula for green rust as [Fe(III)2Fe(II)4(OH)12CO3·2H2O], then the corrosion reaction would be written as:
6 Fe + CO2 + 15 H2O D Fe(III)2Fe(II)4(OH)12CO3·2H2O+ 7 H2 (SOTERM.12)
Roselle (Roselle 2013) determined corrosion rates for steel inundated in brine in the absence of CO2. Based on these rates, a new distribution was presented whose mean value is nearly an order of magnitude less than the previous value determined by Wang and Brush (Wang and Brush 1996). Based on these new corrosion rates, there will still be significant amounts of uncorroded steels and other Fe-base alloys in the repository throughout the 10,000-yr regulatory period.
In reducing environments, reduced iron phases (Fe(II) oxides and zero valent iron) and aqueous ferrous iron will be present. These are all reducing agents towards key actinide species (Table SOTERM-5) and will help establish the predominance of lower-valent actinides in the WIPP. The concentration of ferrous iron could be relatively high in the WIPP brine, although its solubility has not yet been explicitly determined. There are also many potential reactions that could control and/or define the iron chemistry. The expectation is that ferrous hydroxide will control the solubility of iron, leading to a predicted solubility in the range of 10-6 M to 10-4 M for pH between 8.5 and 10.5 (Refait and Génin 1994). A similar range of iron solubility in brines was observed by Nemer et al. (Nemer et al. 2011) in experiments where Fe-hibbingite, Fe2(OH)3Cl, was the solubility controlling phase.
Table SOTERM- 5. Redox Half-Reaction Potentials for Key Fe, Pb, Pu, and U Reactions at 25 oC and I<1 (Morss, Edelstein, and Fuger 2006, Chapter 23)
|
Metal Species Reduced |
Eo (Acidic) (V) |
Eo at pH = 8 (V) |
|
Pb4+ → Pb2+ |
1.69 |
2.47 |
|
PuO2 + → Pu4+ |
1.170 |
0.70 |
|
PuO2 2+ → PuO2 + |
0.916 |
0.60 |
|
Fe(OH)3(s) →Fe2+ |
Not Applicable |
0.1 |
|
FeOOH (s)→FeCO3(s) |
Not Applicable |
-0.05 |
|
UO2 2+ → U4+ |
0.338 |
-0.07 |
|
Pu4+ → Pu3+ |
0.982 |
-0.39 |
|
Pb2+ → Pb |
-0.1251 |
-0.54 |
|
Fe3+→ Fe2+ |
0.77 |
-0.86 |
|
Fe(II)(OH)2 → Fe(0) |
-0.44 |
-0.89 |
|
U4+ → U3+ |
-0.607 |
-1.95 |
Three important reactions of iron are considered. The first is the reaction of metallic iron with carbon dioxide to form strongly insoluble ferrous carbonate. The solubility product of this salt is log K = -10.8 at I = 0 (NIST 2004), and it is much smaller than magnesium carbonate. This suggests that the presence of iron will likely remove CO2 from the repository more effectively than MgO due to its lower solubility product. This reaction is not included in the WIPP PA because the CO2 reacts sufficiently with MgO (so the Fe reaction is not needed) and the DOE does not have sufficient data on the iron carbonation reaction.
The second is the reaction of iron and ferrous ions with the hydrogen sulfide that could be generated in the repository by sulfate-reducing microbes. This will lead to a very insoluble ferrous sulfide precipitate with a solubility product of log Ks = -17.2 (NIST 2004). This helps remove sulfide, which can complex actinides, from brine. This reaction is assumed to occur instantaneously in the PA.
Finally, iron species form strong complexes with organic ligands. The strongest of these complexes is with EDTA. The net effect is that dissolved iron species will compete with actinides for organic ligands, and in many cases out-compete the actinides to counteract the potential enhancement of actinide solubility that would otherwise occur. This reaction is not currently included in the PA because the DOE does not have sufficient data on the reactions that form iron EDTA complexes. Work is currently underway to obtain the necessary thermodynamic parameters for future input into the model.
Lead is present in the repository in the metallic form as part of the waste and waste packaging. The currently anticipated inventory in waste packaging is approximately 8.3 metric tons (Van Soest 2012). The reactivity of zero-valent lead is greatly mitigated by the formation of a thin, coherent, protective oxide, oxycarbonate, chloride, or sulfate protective layer. Metallic lead also reacts slowly with water at room temperature and undergoes corrosion to form oxides and oxyhydroxides. Under slightly alkaline conditions, the hydrolysis of lead leads to formation of a poly-oxyhydroxide cation, [Pb6O(OH)6]4+. The following reactions are possible under WIPP-relevant conditions:
2Pb + O2 D 2PbO (SOTERM.13)
2PbO + H2O + CO2 D (PbOH)2CO3 (SOTERM.14)
Pb + H2O + CO2 D PbCO3 + H2 (SOTERM.15)
Pb + H2S D PbS + H2 (SOTERM.16)
Pb2++ 2Cl- D PbCl2 (SOTERM.17)
Pb2++ SO4 2- D PbSO4 (SOTERM.18)
5Pb2++ PbO + 6OH- D [Pb6O(OH)6]4+ (SOTERM.19)
The corrosion of lead in WIPP-relevant conditions was studied extensively by Roselle (Roselle 2009, Roselle 2010, Roselle 2011a, Roselle 2011b, and Roselle 2013). In these experiments, lead coupons were immersed in the WIPP brines (GWB and ERDA-6) under anoxic conditions and a range of atmospheric CO2 concentrations. Results from multiyear experiments show formation of Pb-Ca carbonate phase at CO2 > 350 ppm. No corrosion product buildup was observed in the absence of CO2; however, coupons were discolored due to the likely formation of lead oxide. Corrosion rates for lead in the absence of CO2 were similar to those measured for steel (Roselle 2013).
The solubility of lead in the WIPP brine is expected to be low, due in part to the passivation process, but also because of insoluble solids formation. Strong oxidants (e.g., radiolysis products) may locally enhance the dissolution of lead, but alkaline brine, which contains chlorides and carbonate/bicarbonate species, will overwhelm radiolytic effects to maintain a low concentration of lead in the brine. In solution, lead will exist as Pb2+ species that are redox-active toward high-valent actinides (see Table SOTERM-5) and will help establish and maintain reducing conditions in the brine.
Lead, as was the case with iron, can influence the redox chemistry (see Table SOTERM-5) and precipitate carbonate and sulfide from the WIPP brine. This leads to a redox chemistry that will help maintain reducing conditions and effectively lower carbonate concentration. Both of these will potentially lower actinide solubility in the WIPP. These impacts are not considered in the WIPP PA due to a lack of sufficient data, and this remains a conservatism in the WIPP model.
Organic chelating agents are used in the processing and cleanup/decontamination of actinides throughout the DOE complex. For this reason, they are often present as co-contaminants with the TRU component in the WIPP waste. Some of these chelating agents strongly complex actinides and have a significant effect on their solubility in brine. In this context, four organic chelating agents-oxalate, acetate, citrate, and EDTA-are tracked as part of the WIPP inventory process, and the potential effects of these complexants on the calculated actinide solubilities were evaluated as part of the CRA-2014 WIPP PA (Brush and Domski 2013a and Brush and Domski 2013b).
The potential concentrations of the key organic ligands in the WIPP used in the CRA-2014 PA were calculated by Brush and Domski (Brush and Domski 2013b) and are based on the 2012 WIPP inventory data (Van Soest 2012). The organic concentrations for the minimum brine volume used in the CRA-2009 PABC and CRA-2014 PA (see section SOTERM-2.2.4) are summarized in Table SOTERM-6. In the WIPP PA implementation, variable brine volume concentrations will be used as described in Brush and Domski (Brush and Domski 2013a).
Table SOTERM- 6. Comparison of the Concentrations of Organic Ligands in WIPP Brine Used in the CRA-2009 PABC and the CRA-2014 PA
|
Organic Ligand |
CRA-2009 PABC Maximum Anticipated Concentration (M)b |
CRA-2014 PA Maximum Anticipated Concentration (M)c |
|
Acetate |
1.94 × 10-2 |
2.30 × 10-2 |
|
Oxalatea |
1.73 × 10-2 |
1.18 × 10-2 |
|
Citrate |
2.38 × 10-3 |
2.33 × 10-3 |
|
EDTA |
6.47 × 10-5 |
7.40 × 10-5 |
|
a - the concentration of oxalate may be limited by its solubility, not inventory, in ERDA-6 brine. b - Brush and Xiong 2005a c - Brush and Domski 2013b |
||
Dissolved metals will compete with the actinides to form organic complexes. As the metals in the repository corrode, additional transition metal ions will dissolve into the brine. These ionic species include iron (Fe) and lead (Pb). Other steel constituents, such as nickel (Ni), chromium (Cr), vanadium (V), and manganese (Mn), may also be present. Additionally, divalent cations in the brine, most importantly Mg2+ and Ca2+, will also form complexes with these chelating agents and compete with the actinide species. The stability constants for Mg2+, Ca2+, Fe2+, Pb2+, and Ni2+ and deprotonation constants for the organic acids are shown in Table SOTERM-7 (NIST 2004). These formation constants, in many respects, follow the same trends as the actinide species with respect to the strength of the complexant (e.g., EDTA > citrate >> oxalate and acetate). When present in high enough concentrations, these metals will compete with the actinide to form complexes and effectively lower the effect of organic complexation on actinide solubility. However, this is not included in the PA and remains as a conservatism in the WIPP actinide concentration model.
Table SOTERM- 7. Apparent Stability Constants for Organic Ligands with Selected Metals (NIST 2004)
|
Organic Ligand |
pKa |
Metal |
Ionic Strength (m) |
log10 β1 |
|
EDTA |
k1 8.86-9.05 k2 6.10-7.02 k3 2.79-2.54 k4 2.05-2.20 |
Fe2+ Ni2+ Pb2+ Mg2+ Ca2+ |
0.1 0.1 0.1 1 1 |
14.3 18.4 18 8.61 9.68 |
|
Citrate |
k1 5.58-5.30 k2 4.25-4.38 k3 2.85-3.06 |
Fe2+ Ni2+ Pb2+ Mg2+ Ca2+ |
0.1 0.1 1.0 0.1 0.1 |
4.4 5.18 4.44 3.43 3.48 |
|
Oxalate |
k1 3.74-4.23 k2 1.15-1.43 |
Fe2+ Ni2+ Pb2+ Mg2+ Ca2+ |
1.0 0.1 1.0 0.1 0.1 |
3.05 4.16 4.20 2.75 2.46 |
|
Acetate |
k1 4.52-4.99 |
Fe2+ Ni2+ Pb2+ Mg2+ Ca2+ |
3.0 0.1 0.1 0.1 0.1 |
0.54 0.88 2.15 0.51 0.55 |
There are two final, but important, observations about the organic chelating agents present in the WIPP. First, they are expected to have very different tendencies toward biodegradation, based on extensive experience with soil bacteria in the literature (Banaszak, Rittmann and Reed. 1998; Reed, Deo and Rittmann 2010). Microbial activity, based on many general observations with soil bacteria, will likely readily degrade citrate, oxalate, and acetate to very low (submicromolar) steady-state concentrations. This important degradation pathway is not as certain for EDTA, which tends to resist biodegradation in most groundwater. These degradation pathways have, however, not been demonstrated for the halophiles typically present in the WIPP, and it is currently assumed in the WIPP PA that no degradation pathways for these organic complexants, microbiological or chemical, exist.
The second important observation is that these chelating agents, under WIPP-relevant conditions, are expected to help establish reducing conditions in the WIPP because they tend to reduce higher-valent actinides. This has been demonstrated in the WIPP brine for Np(V) and Pu(V/VI), but was not observed for U(VI) (Reed et al. 1998). These chelating agents also tend to oxidize III actinides to IV, which would have a beneficial effect on actinide solubility in the WIPP because the actinides in the IV oxidation state are approximately 10 times less soluble than actinides in the III oxidation state. These potentially beneficial effects of organic chelating agents on actinide speciation are also currently not included in the WIPP PA and remain a conservatism in the WIPP model.
The WIPP waste contains a relatively high amount of organic material, since much of the waste is residue from laboratory operations where CPR materials were widely used. Current estimates project over 14,000 metric tons of plastic and cellulosic materials with about 1,250 metric tons of rubber material in the WIPP (Van Soest 2012; Table SOTERM-3). This organic material is important from the perspective of repository performance in that it provides an organic "feedstock" for microbial activity that could lead to gas generation (carbon dioxide, hydrogen, hydrogen sulfide, and possibly methane), as well as degradation products that can complex actinides or form pseudocolloids. CPR degradation is represented in the PA to evaluate these potential impacts on the actinide concentrations and release.
There are three important post-emplacement processes that take place in the WIPP after repository closure. These are metal corrosion, microbiological effects, and radiolysis. Metal corrosion was already discussed as part of the iron chemistry section (Section SOTERM-2.3.4). Microbiological effects and radiolysis are br iefly discussed in this section.
Microbiological processes can have a significant effect on many aspects of subsurface chemical and geochemical processes. This, particularly as it relates to contaminant transport and remediation, has been well established for soil bacteria in low-ionic-strength and near-surface groundwaters (Reed, Deo and Rittmann 2010; Banaszak, Rittmann and Reed 1998). In the WIPP, as a result of the high-ionic-strength brines present, halophilic microorganisms will predominate. In prior recertifications, what was understood about halophilic organisms under WIPP-relevant conditions was established through a series of long-term studies conducted as part of the Actinide Source Term Program (ASTP) project by researchers at Brookhaven (Brush 1990; Francis and Gillow 1994; Brush 1995; Wang and Brush 1996). This, since CRA-2009, was significantly extended by newer studies (Swanson et al. 2012; Swanson et al. 2013a; Swanson et al. 2013b; Swanson and Simmons 2013). Although this new information has increased our understanding of microbial processes in the WIPP, there are no changes to the WIPP microbial model proposed for the CRA-2014.
The potential effects of microbial activity on the fate and transport of actinide metals from deep geological waste repositories have been well described (McCabe 1990; Lloyd and Macaskie 2002; Pedersen 2002; Wang and Francis 2005) and may include 1) gas generation from the degradation of organic waste components, 2) the creation of a reducing environment from oxygen consumption, 3) redox reactions with metals and oxyanions, 4) the generation of organic ligands from the incomplete degradation of organic waste components, and 5) the mobilization of actinides adsorbed onto organism surfaces.
The WIPP PA considers gas generation, since it leads to CO2 formation and increased dissolved carbonate, and biocolloid formation to have the largest potential impact on the mobile concentration of actinides in the source term. Because of the scarcity of data during the time of earlier certification efforts (Barnhart et al. 1978a, Barnhart et al. 1979b, Barnhart et al. 1979c and Barnhart et al. 1979d), there are high levels of conservatism in the current WIPP microbial model and there are no proposed changes to this model in the CRA-2014. This conservatism is attributed to the high uncertainty about microbial processes in hypersaline systems since these are not well studied and because microbial processes attributed to low-ionic strength environments were conferred upon the organisms that inhabit salt-based repositories. In fact, we are finding that organisms indigenous to hypersaline environments, such as the WIPP and its environs, are not as metabolically diverse as is typically seen with their low ionic strength counterparts and are, therefore, far less likely to play a large role in waste transformation. There is still uncertainty about what organisms may predominate should brine inundation occur, but it is becoming clear that non-halophilic organisms introduced with emplaced waste are unlikely to survive the near-field conditions. The microbial model assumptions that are used in the WIPP PA are discussed extensively elsewhere (Appendix PA-2014, Sections 2.1.1 and 4.2.5; U.S. EPA 2006). In this section we provide an updated view of the WIPP microbial ecology, present some new experimental results and discuss these in the context of some of our current PA assumptions. These results increase the DOE's overall understanding of the microbial ecology in the WIPP and suggest that the current WIPP PA assumptions about microbial gas generation are conservatively high.
Hypersaline conditions result in a unique microbial ecology due to the thermodynamic constraints imposed upon the organisms inhabiting such environments. Survival in hypersaline systems depends on an organism's ability to maintain osmotic balance with its external environment (Oren 2006).
If the energetic cost of this maintenance exceeds the benefit of a given metabolic reaction, that reaction will not proceed. As a result, microbial metabolic processes are limited to the following at salt concentrations greater than 2.5 M NaCl, the cut-off for extremely halophilic microorganisms: oxygenic and anoxygenic photosynthesis; aerobic respiration; denitrification; fermentation; manganese, arsenite, and selenate reduction; dissimilatory sulfate reduction with incomplete organic oxidation; methanogenesis from methylated amines; acetogenesis; and chemolithotrophic oxidation of sulfur compounds (see Figure SOTERM-3; Oren 1999 and Oren 2011). All of these processes are either energetically favorable or are performed by organisms that maintain osmotic balance by a less costly strategy.
Figure SOTERM- 3. Approximate Upper Salt Concentration Limits for the Occurrence of Selected Microbial Processes (from Oren 2011). Solid bars are derived from laboratory experimental data using pure cultures; open bars are taken from in situ measurements of possible microbial activity.
Apart from these thermodynamic constraints, the repertoire of potential microbial metabolic pathways within the WIPP is limited even further by 1) physical confinement of the repository without input of exogenous electron acceptors (especially oxygen), moisture (i.e., brine), or light; 2) high ionic strength; 3) high pH; and 4) nonideal substrates. These factors may restrict or effectively eliminate many capabilities.
Thus, microbial activity within the repository should be considered as varying in time and space (Swanson et al. 2012; Swanson and Simmons 2013). Obligate aerobic and extremely halophilic organisms may dominate the initial oxic environment, followed by the low-probability appearance of extremely halophilic anaerobes. In regard to space variation, extreme halophiles will dominate the near-field; while the salinity gradient will dictate the level of halophilism of organisms found within the far-field. Recent work on the characterization of microorganisms at the WIPP has supported these time and space assumptions and will be reviewed below (Swanson et al. 2012; Swanson and Simmons 2013).
Variation in time
Aerobic respiration by haloarchaea will be predominant immediately after repository closure and will remain so until oxygen levels decrease from the corrosion of iron canisters and less importantly, due to microbial activity. Once oxygen has been depleted, nitrate, organic acids, and sulfate will be present as potential electron acceptors.
While most haloarchaea are obligate aerobes, some are capable of nitrate reduction and fermentation of small organics, such as amino acids. However, once conditions become anaerobic, haloarchaeal numbers will decrease and cells will become dormant. The longevity of these organisms entrapped in fluid inclusions or in interstitial brines is well documented; thus, they will be present throughout repository history but are not likely to be active because of unfavorable conditions (Norton and Grant 1988; Mormile et al. 2003; Schubert et al. 2009; Schubert et al. 2010).
Halophilic, aerobic fungi and bacteria have also been isolated from WIPP halite (Swanson et al., 2012; Swanson and Simmons 2013; Gunde-Cimermann et al. 2009). These organisms may survive the early oxic and moist conditions of the repository prior to inundation, but are unlikely to survive in stringent WIPP brines that exceed either their sodium or magnesium tolerances. In anaerobic enrichments of WIPP halite, fungal hyphae did not elongate and spores did not grow, and eventually the fungi died off (Swanson and Simmons 2013).
Bacteria have only been isolated from WIPP halite under aerobic, low-salt conditions, although they may grow in up to 3.4 M NaCl (Swanson et al. 2012). A halophilic denitrifier, Halomonas sp. WIPP 1A, was isolated from previous studies, but its actual source is unknown (Francis et al. 2000). Sulfate reducers (Bacteria) have thus far not been found in subsurface halite. Gillow and Francis (Gillow and Francis 2006) noted a sulfide precipitate in their long-term incubations, which they attributed to the presence of sulfate-reducing bacteria (SRB); however, these incubations were also inoculated with brine lake sediment, the most likely source of these organisms. SRBs have been found in other hypersaline environments (i.e., brine lakes, solar salterns; Porter et al. 2007; Sørensen et al. 2009). Their presence in seeps or in the underlying Castile formation brines is unknown. Sulfate is present in Castile brine and is also formed from the dissolution of anhydrite present in the halite interbeds.
Variation in space
The variation of microbial communities in space concerns the near-field versus intermediate-field versus far-field and reflects the variation in ionic strength in these spaces. With differing ionic strength comes differing community compositions and, hence, different metabolic potential.
Extremely halophilic archaea and some few bacteria may survive at the NaCl concentrations expected in the near-field. Incubations of WIPP halite under high-salt conditions (4.7 M NaCl) yielded only archaeal isolates (Swanson et al. 2012), and these survive in the WIPP brine. The intermediate-field, an area of hypothetical mixing of the repository soup and Culebra groundwater, should support the growth of these same and other haloarchaea and also halophilic bacteria and fungi. The Culebra is considered to be the most likely pathway for actinide migration from the repository, in the low-probability event of a breach into the WIPP horizon (U.S. DOE 2011), and is therefore considered as the far-field. This space has been shown to be dominated by a range of moderately halophilic and halotolerant bacteria, whose diversity decreases as ionic strength increases (Swanson and Simmons 2013).
This distinction in space is important in that waste transformation will occur more readily in the presence of bacteria than archaea. Metabolic activities shown to occur in the far-field include aerobic respiration, fermentation, nitrate reduction, iron reduction, and sulfate reduction; while still other processes are energetically feasible-reduction of other oxyanions, chemolithotrophy (oxidation of ammonium, sulfide), methanogenesis at lower salt concentrations, and methanotrophy (Oren 1999, Oren 2008, and Oren 2011; Swanson et al. 2012; Swanson and Simmons 2013).
Influence of Substrate
Ideal substrates for haloarchaea include small organics, such as amino acids, acetate, glucose, and often citrate (Oren 2006). Organic complexing agents-acetate, oxalate, citrate, and EDTA-will be the predominant low-molecular-weight carbon substrates, but cellulosics may contribute significantly to the carbon inventory. The availability of these substrates will depend upon their dissolved concentrations in brine, which are expected to be at or below the current inventory-predicted levels (Table SOTERM-6). The WIPP haloarchaea are capable of degrading acetate, citrate, and oxalate in aerobic brines (Swanson et al. 2013a; Swanson et al. 2013b) but there is as yet no evidence for anaerobic degradation. In the far-field, acetate and citrate degraders are present, but the fates of oxalate and EDTA have not been studied (Swanson and Simmons 2013).
Organisms introduced in waste
If soil has been introduced into waste containers, it may contain diverse microorganisms capable of many different types of metabolism, and some may even be halotolerant. Spore-forming organisms may survive in spore form for extremely long periods of time, but they will not likely vegetate. Even a halophilic Virgibacillus sp. isolated from WIPP groundwater was unable to grow at NaCl concentrations above 17.5%, at which point it sporulated (Swanson et al. 2012). Although the survival of soil organisms at expected WIPP ionic strength has not been shown, gas generation was reported in incubations of soil and TRU-simulated waste in brine (Caldwell et al. 1988); however, results were equivocal, and microbes were never looked for in the samples.
In spite of conditions inconducive to significant microbial activity, the theoretical possibility of such an occurrence must be taken into consideration by PA, and the current knowledge of the microbial ecology at the WIPP should not affect these assessments other than to underscore the conservatism of the model.
The generation of gas from the degradation of cellulose in waste repositories is an important process that is difficult to reliably predict. The WIPP PA considers this process to be a guaranteed occurrence, which is a very conservative approach, that proceeds completely to carbon dioxide and water (Appendix PA-2014, Section 4.2.5 ) if the following conditions are met: 1) microorganisms capable of degrading cellulose are present at repository closure, 2) these organisms can survive for a significant length of time, and 3) sufficient water, electron acceptors, and nutrients are available to support their activity (Brush 1995). The reality is that cellulose degradation is a complex process requiring the concerted efforts of many different groups of organisms, few of which are either found or would survive in the WIPP. Additionally, the degradation process differs between aerobic and anaerobic environments, as the organisms within those spaces utilize different mechanisms for hydrolysis (Lynd et al. 2002; Wilson 2011).
Sources of cellulose in hypersaline habitats include dead algal biomass and halophytic debris. Many halophilic microorganisms possessing cellulase activity or capable of growth on cellulosic substrates have been reported. Two fungi isolated from WIPP halite, one with documented ligninolytic capability (Cladosporium; Cronin and Post 1977; Gunde-Cimerman et al. 2009), were capable of growth on Kimwipes and using carboxymethylcellulose (CMC) as the sole carbon source (Swanson and Simmons 2013). Moderately halophilic bacteria, including organisms similar to those isolated from the WIPP environs-e.g., Halomonas sp., Virgibacillus sp., and Salinicoccus sp.-have also exhibited cellulase activity (Rohban et al. 2009), and an extremely halophilic bacterium, Marinimicrobium haloxylanilyticum, was found to degrade CMC in up to 22% NaCl. A mixed culture, presumably archaea, enriched from WIPP halite adhered to and altered Kimwipe fibers, as observed microscopically (Vreeland et al. 1998).
Of the above, the bacteria and fungi are unlikely to survive in the WIPP brines, and all are obligately aerobic. Only one anaerobic, cellulolytic microorganism has been isolated from a hypersaline environment-Halocella halocellulolytica (Simankova and Zavarzin 1992; Simankova et al. 1993). This organism degrades cellulose (filter paper) in concentrations of NaCl up to 20%. Thus, it is unlikely that any significant anaerobic cellulose degradation will occur in the WIPP near-field, but lower salinities may permit utilization in the far-field.
Early studies on gas generation were carried out as part of the Actinide Source Term Program (ASTP) at Los Alamos National Laboratory, and later studies took place at Brookhaven National Laboratory. While inconclusive, these studies support the general opinion that organisms introduced into the WIPP along with emplaced waste will not be able to survive in high ionic strength media.
Later studies showed gas generation from cellulose degradation, and these were used as the basis for the WIPP PA assumptions (Francis and Gillow 1993; Francis et al. 1997; Gillow and Francis 2006). These studies used a mixed inoculum, including brine lake sediment and water, underground brine seep, and halite. Because of the relatively rich inoculum, the rates of gas generation measured can be considered optimistic. Sediments are rich in organisms, including anaerobes, even in hypersaline systems. Even so, these studies provide a more realistic scenario than the earlier studies, especially since expected WIPP conditions were better known at the time of design.
The presence of exogenous cellulolytic bacteria introduced in the waste drums themselves cannot be ruled out and, in fact, these organisms have been detected in simulated waste pits (Field et al. 2010). If any moisture were present in the drums, these organisms may have had a chance to cause initial cellulose breakdown to products more easily metabolized by cellulase-producing bacteria should they come into contact with these by-products during early oxic periods. Again, these organisms are unlikely to survive or be active in brine, although some are likely to be halotolerant.
Cellulase-producing haloarchaea, including Haloarcula, Halobacterium, and Halorubrum spp., have been isolated previously from hypersaline salt lakes and salterns (Birbir et al. 2007). While these organisms are likely to thrive at high ionic strength, their use of cellulose by-products will be limited, once again, to early oxic periods.
Still, complex organics may be more recalcitrant to degradation in anaerobic, hypersaline systems. Cellulose fibers have been preserved in fluid inclusions extracted from WIPP halite, suggesting that this type of high-molecular weight organic is recalcitrant to degradation at high salt concentrations, possibly owing to the lack of ionizing radiation, water available for hydrolysis, and microbial activity (Griffith et al. 2008).
The current PA approach to account for CPR degradation, although there are many reasons that a much lower extent of biodegradation should occur, remains conservative in that it assumes that the biodegradation of all cellulosic and plastic material is guaranteed. This adds to the overall conservatism of the WIPP actinide source term model.
The microbially induced reduction of higher-valent actinides would be an important beneficial effect for the WIPP, in that lower-valent actinide species are less soluble. This has recently been the focus of much research due to its expected role in microbially mediated remediation and containment of subsurface contaminants (Banaszak, Rittman, and Reed 1998; Banaszak et al. 1999; Lloyd, Young, and Macaskie 2000; Reed et al. 2007; Icopini, Boukhalfa, and Neu 2007; Francis, Dodge, and Gillow 2008). For soil bacteria, there is no question that biotic mechanisms that lead to the reduction of actinides exist under a wide range of anaerobic subsurface conditions.
There are, however, very few data concerning metal reduction in hypersaline environments (Sorokin and Muyzer 2010; Emmerich et al. 2012). This is likely due to the low solubility of oxidized metal species in these systems; thus, the data are generally limited to lower ionic strength systems, insoluble metal oxides in sediments, or metals associated with particulate organic matter or associated with microbial mats. The ability of WIPP-indigenous microorganisms to reduce metals is again divided between near-field and far-field spaces.
Both Clostridium spp. and sulfate-reducing bacteria with metal-reducing capability have been detected in the Culebra and could be capable of directly reducing higher-valent actinides in the far-field (Swanson et al. 2012; Swanson and Simmons 2013). Additionally, the indirect reduction of iron by either fermenting Halanaerobium spp. or SRB was shown to precipitate iron-sulfide complexes by lowering the redox potential or reducing sulfate, respectively, in iron-amended enrichment incubations. The reduction of iron by other SRB and fermenters has been shown previously in hypersaline sediments (Emmerich et al. 2012).
In the near-field, however, it is less likely that direct biotic processes will cause actinide reduction since haloarchaea have not been shown to directly reduce metals.
The potentially beneficial effects of bioreduction to lower the solubility of multivalent actinides are not considered in the WIPP PA. There remains high uncertainty about this process for halophilic microorganisms and there is not yet definitive WIPP-specific data to explain and support this reduction pathway.
There is no change from the CRA-2009 in the CPR microbial degradation model proposed in the CRA-2014. There are no new gas generation rates, and the implementation and basis of the gas generation rates has not changed (Appendix PA-2014, Section 4.2.5 ).
Currently, microbial activity is considered in all PA vectors. The presence of this microbial activity means it is assumed that microbes may consume 100% of the cellulosic materials in the repository, and that there is a probability of 0.25 that microbes may consume the plastic and rubber materials. Thus, there is microbial consumption of cellulosic materials, but not of plastic or rubber materials, in 75% of the PA realizations (vectors), and microbial consumption of CPR materials in 25% of the vectors.
Microbial consumption of CPR materials could affect the actinide source term in four ways:
1) Productionof significant quantities of CO2, which could acidify the brine in the absence of an MgO buffer or increase the solubility of actinides by increasing carbonate levels that complex some actinides at the expected mildly alkaline pH
2) Bioreduction of higher-valent actinide species leading to lower-valent, less-soluble actinide species
3) Degradation of solubilizing organic ligands, leading to lower actinide solubility
4) Increased biomass that may lead to the formation of microbial colloids that increase the amount of actinide pseudocolloids in the brine
The effect of CO2 production is discussed in this section. The remaining three effects are implicitly considered in the analyses that address the oxidation-state distributions (Section SOTERM-4.3), the effects of organic ligands (Section SOTERM-2.3.6), and the effects of colloids (Section SOTERM-3.9). The simplifications used in the PA calculations for all four of these effects are discussed at the end of this section.
Microbial activity, if it occurs to a significant extent in the WIPP, would consume CPR materials by the following sequential reactions (Brush 1990; Francis and Gillow 1994; Brush 1995; Wang and Brush 1996; Francis 1998):
C6H10O5 + 4.8H+ + 4.8NO3 - ® 7.4H2O + 6CO2 + 2.4N2; (SOTERM.20)
C6H10O5 + 6H+ + 3SO4 2- ® 5H2O + 6CO2 + 3H2S; (SOTERM.21)
C6H10O5 + H2O ® 3CH4 + 3CO2. (SOTERM.22)
Methanogenesis, described by reaction SOTERM.22, is not included as a degradation pathway because it is assumed that the sulfate present in the DRZ is always available. This exclusion is considered a conservative assumption relative to the amount of carbon dioxide that could be produced. In effect, the CRA-2014 PA assumes that an excess of sulfate is always available to sustain sulfate-reduction as a mode of respiration. When unlimited sulfate is available from natural sources in the host rock, 4% of the gas generation occurs through denitrification and 96% occurs by way of sulfate reduction. The omission of methanogenesis is now further supported by the fact that this process has been shown unfavorable at the ionic strengths expected in the WIPP (Oren 2011, also see discussion in Section SOTERM-2.4.1.1). Microbial consumption of CPR materials, therefore, is assumed to produce significant quantities of CO2, which could in turn acidify any brine present in the repository and increase the solubilities of the actinides relative to those predicted for neutral and mildly basic conditions. Therefore, the DOE is emplacing MgO in the repository to decrease actinide solubilities by consuming essentially all of the CO2 that could be produced by microbial consumption of CPR materials, and by buffering (controlling) the fCO2 and pH within ranges that are favorable from the standpoint of actinide speciation and solubility (see Section SOTERM-2.3.2).
Three effects of microbial consumption of CPR materials are recognized in the system performance modeling. A simplification has been made so the effects will be time-independent after 100 years. These effects are
1. CO2 production: With the addition of excess MgO, the effects of CO2 production are minimized, and it is assumed that the system may be modeled using the brucite-hydromagnesite (Mg5(CO3)4(OH)2 ×4H2O) buffer.
2. Redox effects: After 100 years, the repository will have a reducing environment. This is, in part, established by the postclosure microbial consumption of oxygen, but is also due to the corrosion of steel. This combined effect leads to the formation of an anoxic reducing environment in the WIPP.
3. Biocolloid formation: Production of microbial colloids is possible and may contribute to the formation of colloidal species that add to the actinide source-term concentration in DBR release.
Radiolysis effects in the WIPP are caused by the interaction of ionizing radiation and particles (neutrons, α, β, and γ) with the gases, brines, and materials present in the repository. These effects have not been extensively studied under WIPP-related conditions, but there is a fairly good general understanding of their extent and nature. For most conditions expected in the WIPP, radiolytic effects are predicted to be transient and insignificant. In this context, there is a recognition that although radiolysis can lead to localized conditions and effects that could oxidize multivalent actinides, the brine chemistry, metal corrosion, and microbiological activity will combine to very rapidly overwhelm these effects. For this reason, radiolysis effects on actinide solubility are not explicitly included in the WIPP PA to calculate actinide concentrations. More specifics on the overall mechanisms, brine radiation chemistry, and potential radiolytic effects on actinide speciation are given in this section.
There are no new data on the radiolysis of brine systems since the CRA-2009. Radiolytic effects continue to be low in importance for transuranic waste under WIPP-relevant conditions and data obtained (see section SOTERM-3.6) on Pu-Fe systems show that the iron chemistry and expected reducing conditions prevail over radiolytic processes.
The radiolysis of high-ionic-strength brine systems has not been extensively studied, but some studies exist (Büppelman, Kim, and Lierse 1988; Kim et al. 1994; Kelm, Pashalidis, and Kim 1999; Ershov et al. 2002). The many components in the brine systems of interest to the WIPP will lead to relatively complex radiation chemistry and the formation of numerous transients and free radicals.
In contrast to this, the radiation chemistry of pure and dilute aqueous systems has been extensively investigated, and detailed reviews of this research have been published (Draganic and Draganic 1971; Spinks and Woods 1990). The irradiation of pure water leads to the formation of molecular hydrogen peroxide (H2O2) and hydrogen (H2). These molecular yields are relatively insensitive to a wide range of conditions in dilute systems for a given type of ionizing radiation. Molecular yields are GH2 = 0.45 molecule (molec)/100 electron-volt (eV) and GH2O2 = 0.7 molec/100 eV for low Linear Energy Transfer (LET) ionizing radiation (β, and γ) and GH2 = 1.6 molec/100 eV and GH2O2 = 1.5 molec/100 eV for high LET radiation (α and neutrons). The radiolytic formation of hydrogen in the WIPP brine due to self-irradiation effects of 239Pu was established and a molecular yield of GH2 = 1.4 molec/100 eV was measured (Reed et al. 1993). This yield is consistent with the high LET literature, even though the irradiations were performed in brine.
The high concentrations of electron and free radical scavengers present in the WIPP brine have a pronounced effect on the radiation chemistry. Most importantly, halides react with the hydroxyl radical (OH×) or act as scavengers (such as Cl- or Br-) to gradually lower the molecular yield of H2O2 as the concentration of the scavengers is increasing (Kelm, Pashalidis, and Kim 1999). In this context, oxidizing transient species are "chemically" stored as oxychlorides and oxybromides, leading to a shift towards more oxidizing conditions. Figure SOTERM-4 gives an overview of the radiolytic pathways and mechanisms that are likely (Buppelmann, Kim, and Lierse 1988). In NaCl brine, the formation of chloride species (ClO-, HOCl, Cl2, and Cl3
-) is favored, instead of H2O2 (Büppelmann, Kim, and Lierse 1988).
Figure SOTERM- 4. NaCl Brine Radiolysis Species and Suggested Mechanism of Production. The formation of chloride species (ClO-, HOCl, Cl2, and Cl3 -) is favored instead of H2O2 (based on data in Büppelmann, Kim, and Lierse 1988).
Kelm, Pashalidis, and Kim (Kelm, Pashalidis, and Kim 1999) showed that the formation of hypochlorite ion increases with the chloride concentration and the dose (Figure SOTERM-5) in NaCl brine. The authors found that in solutions containing 37 gigabecquerel (GBq)/liter (L) of 238Pu, the hypochlorite concentration increases with time (dose) and appears to approach a steady state (see Figure SOTERM-5). At a constant dose rate, the maximum hypochlorite concentration depends on the chloride concentration. It was also observed that hypochlorite ion generation was negligible when chloride concentrations were smaller than 2 M.
Figure SOTERM- 5. Radiolytic Formation of Hypochlorite Ion in Solutions of Various NaCl Concentrations at a Constant Alpha Activity of 37 GBq/L at pH~12 (based on data in Kelm, Pashalidis, and Kim 1999)
In the WIPP brine, however, some solutes other than chloride may play a role. Ershov et al. (Ershov et al. 2002) showed that small amounts of bromide in natural brines under radiolysis can give Cl2 -, ClBr-, and Br- radical anions at the radical step, and then mixed halogen molecules and trihalide ions by radical recombination at the molecular step (Ershov et al. 2002). The hydrolysis of mixed halogen molecules can then result in the formation of hypobromite (OBr-) (acidic form: hypobromous acid [HOBr]), a starting substance to more stable bromates of higher oxidation state (Ershov et al. 2002).
Some WIPP-specific experiments were performed to establish the key radiolytic product in GWB and ERDA-6 brine (Lucchini et al. 2010a). This study confirms that hydrogen peroxide (H2O2) and hypochlorite ion (OCl-) are unstable in these WIPP brines, due in part to metallic impurities in the brine. There was, however, an accelerated decomposition of these species when bromide (Br-) was present, which is the case for both ERDA-6 and GWB brines. Here, OCl- readily and stoichiometricly reacted with Br- to form hypobromite ion (OBr-), which appeared to be the most important radiolytic transient observed under these conditions. OBr-, like OCl-, is also an oxidizing species (Eº=0.76V), that will likely lead to the oxidation of multivalent actinides in the WIPP, but this reactivity has not been established experimentally under representative WIPP conditions (Lucchini et al. 2010a).
In the WIPP, most of the brine radiolysis is caused by the deposition of alpha particles from the TRU isotopes present in the WIPP waste. The range (distance traveled until the alpha particle's energy is lost) of these alpha particles is very short (<40 microns) and radiolysis of the brine solution will take place at the solid-liquid interface. Locally, the concentration of oxidative radiolytic products of brine, such as hypochlorite, chlorite, chlorate, and products of their reaction with brine components (e.g., hypobromite) may be high, and they may directly interact with the radioactive surface. These "very-near" radiolytic effects, however, are expected to be quickly mitigated by the bulk brine chemistry and the reaction of reducing agents (e.g., reduced iron) with the oxidizing molecular products formed.
A buildup of oxidizing radiolytic products in brine may increase the redox potential of the brine (Büppelmann, Kim, and Lierse 1988), and consequently directly generate higher-valent actinide species. Alternatively, these radiolytic products could be inserted into some solid actinide phases. For example, Kim et al. (Kim et al. 1994) studied the solubility of schoepite, (UO2)(OH)2 ×xH2O, with hypochlorite ion in 0.1M NaCl at 25 °C (77 °F), in CO2-free atmosphere (Kim et al. 1994). Their X-Ray Diffraction (XRD) patterns of the residual precipitates showed the introduction of the hypochlorite ion in precipitates. Kim et al. (Kim et al. 1994) observed that the presence of hypochlorite ion in the initial schoepite structure enhanced the solubility of the solid 10 to 100 times in the range of pH 6.0-9.8, compared with its solubility in the absence of hypochlorite ion (Kim et al. 1994). However, this effect was reduced when the molar ratio [ClO-]/[UO2 2+] increased. This scenario is unlikely to occur in the WIPP because the potential buildup of oxidizing radiolytic products generated in brine is readily overwhelmed by the overall reducing capacity of the site (reduced metals and microbial processes).
The buildup of oxidizing radiolytic products due to brine radiolysis has also been shown to significantly affect the solution chemistry of Am. For example, Am(III) was oxidized to the more soluble forms of Am, namely AmO2 + and AmO2 2+ (Magirius, Carnall, and Kim 1985; Katz, Seaborg, and Morss 1986; Stadler and Kim 1988; and Meyer et al. 2002). Magirius, Carnall, and Kim (Magirius, Carnall, and Kim 1985) reported on the radiation effects exerted upon a 5 M NaCl solution at the pH 8 to 9 range using precipitated Am(OH)3 at a concentration of 1.03 × 10-3 M (1.07 curie [Ci]/L). They observed that the precipitate began to show discoloration, changing from pink Am3+ to brown AmO2 +, within 24 hours (h), with quantitative oxidation of all the Am to AmO2 + within 1 week. Because Pu is more readily oxidized than Am, the expectation is that Pu could also be oxidized in irradiated brine. The metastability of Pu(VI) in the WIPP brine when no reducing agents were present was established and attributed to self-radiolysis effects of the 239Pu isotope used (Reed, Okajima, and Richmann 1994; Reed et al. 2006).
Stadler and Kim (Stadler and Kim 1988) also report the existence of higher oxidation states of Am, due to self radiolysis. Solubility experiments on Am(OH)3(solid[s]) in 3 M NaCl resulted in much higher Am concentrations than was calculated from the solubility product. This difference was assigned to the radiolytic oxidation of Am3+ to AmO2 +. Spectrophotometric evidence of AmO2 + species in solution was reported. The authors report the value of log10KS,0 = -9.3 ± 0.5 for the reaction
AmO2OH(s) D AmO2 + + OH- (SOTERM.23)
The solubility product of AmO2OH(s) is in general agreement with other solubility studies on different pentavalent actinides.
These results show there is clearly a potential for oxidized, higher-valent actinides to form in brine when no reducing agents are present. This, however, needs to be interpreted in the context of the strong reducing agents and processes that will predominate in the WIPP, such as bioreduction (Section SOTERM-2.4.1.2), iron reduction (Section SOTERM-2.3.4), and reduction by organic complexants (Section SOTERM-2.3.6). The WIPP-specific data show that the presence of reduced iron (Fe(II/0)) leads to a rapid reduction of Pu(VI) to Pu(IV) species under a wide range of anoxic conditions (Reed et al. 2006; Reed et al. 2009; Section SOTERM-3.6). These results are expected to extend to the Am(V) system, since this species is more readily reduced than Pu(V/VI). Reduced iron will also react with radiolytically generated oxidizing species, such as hypochlorite or hypobromite, to prevent their buildup in the brine solution with time. In summary, these WIPP-specific results show that the reductants present in WIPP waste (reduced metals and organics) will overwhelm potential radiolytic effects under the expected conditions in the WIPP, and a significant and sustained radiolytic enhancement of actinide solubilities is not predicted.
The speciation of actinides under WIPP-relevant conditions defines the source term for actinide release from the WIPP in release scenarios where dissolved actinide concentrations are important (e.g., DBR and transport through the Salado or Culebra). The key factors that establish the concentrations of dissolved actinides under subsurface conditions are known. The most important of these factors for the WIPP repository are:
1. Actinide redox chemistry is a critical factor in establishing the concentration of actinides in brine. The solubility of reduced actinides (III and IV oxidation states) is significantly lower than oxidized forms (V and/or VI). In this context, the reduced-metal chemistry and microbial processes that establish and maintain reducing conditions in the WIPP are important.
2. The complexation of each actinide species is a critical factor in defining its solubility. For a given oxidation state, the inorganic and organic complexes present will define the solubility of the actinide. These complexants are in the preemplacement environment, are part of the TRU waste that is emplaced, or are produced as a result of subsurface processes, most notably microbial and corrosion processes.
3. Intrinsic and pseudoactinide colloid formation is a critical factor in defining the overall solution concentration of each actinide. The contribution of actinide colloids to the concentration of actinides in the WIPP is predicted to be significant. Many of the key TRU species in their expected oxidation states tend to form colloids or strongly associate with the non-actinide colloids present (e.g., microbial, humic and mineral).
The WIPP PA approach that was established in the initial WIPP license application (U.S. DOE 1996), and continued through the CRA-2014 PA calculations (Camphouse et al. 2013), accounts for all three of these key factors.
The PA concept of actinide speciation in the WIPP is well grounded in what has been observed for actinide contaminants in near-surface groundwater. In natural systems, the following inorganic ligands are potentially important complexants of radionuclides in solution: CO3 2-/HCO3 -, OH-, C1-, SO4 2-/S2-, fluoride (F-), and phosphate. Additionally, anthropogenic and bioderived chelating agents can strongly bind actinide species and will compete with the inorganic complexants present. Lastly, the tendencies of actinides to form intrinsic colloids and strongly associate or bind with colloidal particles are also well established. The relative importance of these complexants and processes depends on the pH, radionuclide oxidation state present, the presence of other metals, and the relative ligand concentrations. There are a number of general reviews on various aspects of actinide environmental chemistry (Allard 1982; Choppin, Liljenzin, and Rydberg 2004 [pp. 94-112]; Clark, Hobart, and Neu 1995; Banaszak, Rittmann, and Reed 1998; Runde 2000; Nitsche et al. 1992; Reed, Deo and Rittmann 2010; Runde and Neu 2010).
For the anoxic, reducing, and mildly basic brine systems expected in the WIPP, the most important inorganic complexants are expected to be carbonate/bicarbonate and hydroxide. There are also important organic complexants that coexist in TRU waste with the potential to strongly influence actinide solubility. In this context, the relative importance of actinides and overall oxidation state with respect to their potential release from the WIPP is:
Actinides: Pu ≈ Am >> U > Th >> Np ≈ Cm (SOTERM.24)
Actinide Oxidation State: An(III) > An(IV) >> An(VI) >> An(V) (SOTERM.25)
In the CRA-2014 PA (Appendix PA-2014, Section 8.4 ), the contribution of Pu, Am, U, Th, Cm, and Np is expressly considered, although only Pu and Am contribute significantly to TRU release from the WIPP. The III oxidation state is the most important oxidation state based on current WIPP PA assumptions that Am always exists in the III state, Pu exists in the III state in ~50% of the vectors, and the III oxidation state is more soluble than the IV (see Section SOTERM-4.0 and Tables SOTERM-20 and SOTERM-24).
In this section, an update of the literature and a summary of new WIPP-specific data is provided (when available) for all the actinides that contribute in one way or another to PA. Section SOTERM-3.1 gives a summary of changes since the CRA-2009 and CRA-2009 PABC; Section SOTERM-3.2 gives an overview of the projected and current inventory of actinides in the WIPP; Section SOTERM-3.3, Section SOTERM-3.4, Section SOTERM-3.5, Section SOTERM-3.6 and SOTERM-3.7 contain an overview of the relevant environmental chemistry and WIPP-specific results for Th, U, Np, Pu, and Am/Cm, respectively; Section SOTERM-3.8 pertains to the complexation of actinides by organic chelating agents in the WIPP; and Section SOTERM-3.9 provides an overview of the potential for the formation of actinide colloids in the WIPP. An up-front overview of the current assumptions and understanding of WIPP actinide chemistry is given in Table SOTERM-8. The PA implementation of this actinide environmental chemistry is discussed in Section SOTERM-4.0 and Section SOTERM-5.0.
Overall, there are few significant changes in the CRA-2014 general approach and assumptions used to understand and predict actinide behavior in the WIPP from a PA perspective. The following key assumptions are continued:
· Oxidation state distributions for the TRU actinides, and correspondingly, assumptions regarding their solubility calculations using redox-invariant analogs, have not changed.
· The approach used to calculate solubilities for Pu and Am oxidation states, which are the key actinides from the perspective of PA, have not changed. EQ3/6, rather than FMT, however, is now being used to calculate these solubilities with the WIPP actinide database.
· Inventory assumptions regarding the amounts of organic chelating agents and actinides in TRU waste are being updated annually.
· The WIPP colloidal model that accounts for intrinsic, mineral fragment, microbial and humic colloidal enhancements has not changed.
Table SOTERM- 8. Overview of the WIPP PA View/Role and Relevant Environmental Chemistry of the Key Actinide Species in the WIPP (References for Each Actinide are Provided in the Following Sections)
|
Actinide |
WIPP PA View/Role |
Environmental Chemistry |
|
Thorium |
Not a TRU component. Currently included in PA calculations, but not a significant contributor to actinide release. Used as an oxidation-state invariant analog for the IV actinides. Th data are used in EQ3/6 to calculate the solubility of Pu(IV), Np(IV), and U(IV). |
Exists as Th4+ complexes and is sparingly soluble under a wide range of environmental conditions. Th has a high tendency towards intrinsic colloid formation. |
|
Uranium |
Not a TRU component. Potentially useful as a VI analog for Pu(VI) species. Currently, U is conservatively assumed to be U(VI) in 50% of the PA vectors (set at a 1 mM solubility) and U(IV) in 50% of the PA vectors. It is not predicted to be a significant contributor to actinide release (based on Ci). |
Exists as UO2 2+ and U4+ species that are strongly correlated with redox conditions. Can form highly insoluble U(VI) and U(IV) phases. Can persist up to mM concentrations in near-surface groundwater. |
|
Neptunium |
TRU component. Currently included in the PA calculations, but not a significant contributor to actinide release. Assumed to be IV in 50% of the PA vectors and V in 50% of the PA vectors. Expected to predominate in the IV oxidation state under the conditions expected in the WIPP. |
Mobile and relatively soluble as the NpO2 + species under oxidizing conditions. Is fairly insoluble and immobile as Np4+ under reducing conditions. |
|
Plutonium |
TRU component. Major contributor to actinide release calculations. Assumed to be IV in 50% of PA vectors and III in the other 50% of PA vectors. |
Relatively immobile and insoluble as a subsurface contaminant. Persists as Pu4+ except under biomediated, strongly reducing conditions where Pu3+ species may be formed. If transported, this will likey be primarily through colloidal mechanisms. |
|
Americium |
TRU component. Major contributor to actinide release calculations. Exists in the III oxidation state in all vectors and its thermodynamic data are used by EQ3/6 for all III oxidation state calculations. Significant colloidal contribution due to strong association as a pseudocolloid. |
Relatively immobile and insoluble as a subsurface contaminant. Persists as Am3+ complexes under a wide range of environmental conditions. |
|
Curium |
Small quantities of 243Cm, 245Cm, and 248Cm are present in the WIPP. 244Cm, although present, is not a TRU waste component due to its <20 year half-life. These are very minor contributors to actinide release. Chemistry is analogous to Am(III). |
Not a very significant concern as a subsurface contaminant. Has the same chemistry as Am, so it will persist as a Cm3+ species. |
|
Organic Chelating Agents |
The effects of EDTA, citrate, oxalate, and acetate on actinide solubility are considered in the WIPP PA. These are present in the WIPP waste and it is assumed that they are neither destroyed nor created by WIPP-relevant subsurface processes. |
EDTA can persist under a wide range of environmental conditions and strongly chelates actinides. Citrate, oxalate, and acetate will likely be degraded due to microbial activity. |
|
Actinide Colloids |
Intrinsic and pseudocolloids with actinides are formed. These are accounted for in the WIPP PA and add to the conservatism of the actinide concentrations calculated. |
Importance and role of An colloid-facilitated transport are the subject of much ongoing debate. The key issue within the WIPP is the potential contribution of colloids to the actinide source term and not their ability to facilitate actinide transport. |
There are new data, within and outside the WIPP project, that continue to support and/or expand the robustness of the current PA assumptions. The most important of these are:
· New WIPP-specific data that confirm the predominance of lower-valent plutonium in long-term, iron-dominated brine systems.
· The solubility of An(IV) in simulated WIPP brines over a wide range of conditions was experimentally determined using Th(IV) as an analog for Pu(IV). These data support current PA solubilities for the IV actinides.
· The effect of the complexation of organic chelating agents on actinide (III/IV) oxidation states was experimentally determined. Relatively strong complexation effects are noted with An(III) that is consistent with current WIPP modeling.
· The solubility of U(VI) as a function of carbonate was determined and shown to be well below the current EPA-set limit of 1 mM.
· The colloidal enhancement parameters were re-evaluated and new parameter recommendations were made. Experiments specific to intrinsic, microbial, and to a lesser extent mineral fragment colloids are reported. These data, although incomplete, provide stronger supporting data for the current WIPP colloid model. The specific changes are described in more detail in Section SOTERM-3.9.
· A variable brine volume approach was implemented in PA to calculate actinide solubility. This extends the minimum brine volume approach used in CRA-2009.
The actinide inventory for the WIPP, based on the Performance Assessment Inventory Report - 2012 (Van Soest 2012), is given in Table SOTERM-9. This is the inventory used in CRA-2014. Also included in this table is the calculated inventory-limited solubility of the various actinides and radionuclides considered by the WIPP PA.
Over long time frames, only Pu and Am are expected to make a significant contribution to releases from the WIPP (see time profile in Table SOTERM-10), although the relative contribution of Am decreases significantly after 1000 years due to its half-life. Curium (Cm), which is predominantly present as 244Cm, is well below the calculated solubility for III actinides when fully dissolved and, with its very short half-life (18.11 years), will not be important beyond the 100-year period of institutional control. Although some cesium (Cs) and strontium (Sr) is initially present in the WIPP, these fission products can only contribute significantly to the overall release from the WIPP for the first 100 years of repository history and are not significant beyond the period of institutional control.
Table SOTERM- 9. WIPP Radionuclide Inventory (Van Soest 2012) Decay-Corrected to 2033. This Inventory was used in the CRA-2014 PA Calculations.
|
Selected Radionuclides |
Activity (Ci) |
Amount (kg) |
Element-Specific Inventory (all reported isotopes) |
Inventory-Defined Solubility Limita (M) |
|
Actinides |
||||
|
229 Th |
1.40 |
6.57×10-3 |
7.04 Ci 1.35×104 Kg |
>> Solubility |
|
230 Th |
4.14 |
0.2 |
||
|
232 Th |
1.50 |
1.35×104 |
||
|
233 U |
139 |
14.2 |
528 Ci 2.26×105 Kg |
>> Solubility |
|
234 U |
242 |
38.3 |
||
|
235 U |
76.4 |
3.49×104 |
||
|
236 U |
5.44 |
83.2 |
||
|
238 U |
64.8 |
1.91×105 |
||
|
237 Np |
23.2 |
32.5 |
23.2 Ci 32.5 Kg |
8 ´ 10-6 M (≥ projected solubility) |
|
238 Pu |
6.01×105 |
34.7 |
2.02×106Ci 1.20×104 Kg |
>> Solubility |
|
239 Pu |
5.74×105 |
9.13×103 |
||
|
240 Pu |
1.75×105 |
762 |
||
|
241 Pu |
6.63×105 |
6.38 |
||
|
242 Pu |
8.09×103 |
2.04×103 |
||
|
244 Pu |
0.0101 |
0.567 |
||
|
241 Am |
7.05×105 |
203 |
7.05×105 Ci 203 Kg |
5 ´ 10-5 M (≥ projected solubility) |
|
243 Am |
51.2 |
0.254 |
||
|
244 Cm |
9.97×103 |
0.122 |
9.97 ×103 Ci 0.122 Kg |
~ 3 x 10-8 M |
|
Fission Productsb |
||||
|
137 Cs |
2.35×105 |
2.67 |
2.35 ×105 Ci 2.67 Kg |
1 ´ 10-6 M |
|
90 Sr |
2.09×105 |
1.51 |
2.09×105 Ci 1.51 Kg |
1 ´ 10-6 M |
|
a Moles in the inventory divided by the minimum brine volume (17,400 m3) b Fission products are not TRU, but are considered in the PA to calculate overall release |
||||
Table SOTERM-10. Time-dependence of Radionuclide Inventory (Van Soest 2012)
|
Element |
2033 (0 years) Ci (Kg) |
2133 (100 years) Ci (Kg) |
3033 (1000 years) Ci (Kg) |
12033 (10,000 years) Ci (Kg) |
|
Th |
7.04 (1.35×104) |
8.52 (1.35×104) |
22.5 (1.35×104) |
127 (1.35×104) |
|
U |
528 (2.26×105) |
645 (2.26×105) |
746 (2.26×105) |
769 (2.28×105) |
|
Np |
23.2 (32.5) |
44.8 (62.9) |
140 (197) |
170 (238) |
|
Pu |
2.02×106 (1.20 ×104) |
1.03×106 (1.19×104) |
7.24×105 (1.16E4) |
5.00×105 (9.12×103) |
|
Am |
7.05×105 (203) |
6.20×105 (179) |
1.47×105 (42.4) |
21.1 (0.0994) |
|
Cm |
9.97×103 (0.122) |
216 (2.65×10-3) |
2.32×10-13 (2.84×10-18) |
0.00 (0.00) |
|
Cs |
2.35×105 (2.67) |
2.33×104 (0.265) |
2.17×10-5 (2.46×10-10) |
0.00 (0.00) |
|
Sr |
2.09×105 (1.51) |
1.78×104 (0.129) |
4.21×10-6 (3.05×10-11) |
0.00 (0.00) |
Th is not a TRU component, although an estimated 13.5 metric tons of Th will be in the WIPP. The release of Th as the 230Th isotope was calculated in the CRA-2014 PA and does not significantly contribute to the overall release of activity from the WIPP. Th is, however, important for the WIPP in that it is used as a redox-invariant analog for the IV actinides (Pu(IV), Np(IV), and U(IV)), and Th complexation data are used in the EQ3/6 code for the An(IV) solubility calculations (Section SOTERM-4.1).
Th, under a wide range of conditions, has one stable oxidation state in aqueous solutions: the Th4+ tetravalent ion. For this reason, the environmental chemistry of Th is understood from the perspective of the solubility and complexation of this species, which is also the species expected to be present in the WIPP environment when DBR and transport release scenarios are important.
Other oxidation states for Th in aqueous systems have been reported. Klapötke and Schulz (Klapötke and Schulz 1997) suggested a Th3+ species as a somewhat stable species in slightly acidic solution but this is not correct; it has been discounted because the proposed reaction for the species' formation is shown to be thermodynamically impossible, and the azido-chloro Th4+ complex was incorrectly assigned to the Th3+ species (Ionova, Madic, and Guillaumont 1998).
The hydrolysis of Th4+, as is true for all An(IV) species in the WIPP, is complex and a critically important interaction in defining the overall solubility of Th. This was recently investigated by Ekberg et al. (Ekberg et al. 2000), Rai et al. (Rai et al. 2000), Moulin et al. (Moulin et al. 2001), and Okamoto, Mochizuki, and Tsushim (Okamoto, Mochizuki, and Tsushim 2003), and was critically reviewed by Neck and Kim (Neck and Kim 2001) and Moriyama et al. (Moriyama et al. 2005). The authors have proposed a comprehensive set of thermodynamic constants that extends to all tetravalent actinides. The solubility products were determined for amorphous (am) Th(OH)4 (Neck et al. 2002; Altmaier et al. 2005 and Altmaier et al. 2006) and for crystalline ThO2 (Neck et al. 2003), as well as for specific ion interaction theory parameters (Neck, Altmaier, and Fanghänel 2006). The thermodynamic stability constants are listed in Table SOTERM-11.
Table SOTERM- 11. Thermodynamic Stability Constants for Key Th Hydrolytic Species
|
Hydrolytic Reaction/Species |
Stability Constant |
|
Mononuclear Species |
|
|
Th(OH)4, am D Th4+ + 4OH- Th(OH)4, cr D Th4+ + 4OH- Th4+ + OH- D Th(OH)3+ Th4+ + 2OH- D Th(OH)2 2+ Th4+ + 3OH- D Th(OH)3 + Th4+ + 4OH- D Th(OH)4,aq |
log Ks,am = -47.8 ± 0.3 log Ks,cr = -53.2 ± 0.4 log β0 1 = 11.8 ± 0.2 log β0 2 = 22.0 ± 0.6 log β0 3 = 31 ± 1 log β0 4 = 38.5 ± 1 |
|
Polynuclear Species |
|
|
4Th4+ + 12OH- D Th4(OH)12 4+ 6Th4+ + 15OH- D Th6(OH)15 9+ |
log β0 4,12 = 141 log β0 6,15 = 176 |
Discrepancies in the ThO2(cr) solubility were recently studied (Vandenborre et al. 2010) and assigned to the different forms of material present: bulk ThO2(cr) grains (80%) and ThOx(OH)y(H2O)z(s) grain boundaries (20%). The hydrated material may originate from the initial grain-boundary oxide materials, which are more sensitive to humidity than the bulk materials. The solubilities of these two phases are quite different and together with the "local solubility" (the most active sites) were used to explain the discrepancies noted.
The presence of carbonate in solution greatly increases the solubility of thorium dioxide (ThO2). An increase by one order of magnitude of the carbonate concentration in the range of 0.1 - 2 M leads to a five-order-of-magnitude increase in the Th(IV) solubility due to the formation of mono- and penta-carbonate complexes. Östhols, Bruno, and Grenthe (Östhols, Bruno, and Grenthe 1994) proposed the following equilibrium reactions and the corresponding stability constants:
ThO2 + H+ + H2O + CO3 2- D Th(OH)3 CO3 - log K131 = 6.11 ± 0.19 (SOTERM.26)
ThO2 + 4H+ + 5 CO3 2- D Th(CO3)5 6- + 2H2O log K105 = 42.12 ± 0.32 (SOTERM.27)
This speciation scheme, however, was criticized in recent work (Altmaier et al. 2005) because it overpredicts the dependency of Th solubility on carbonate and underpredicts the effect of hydrolysis at higher pH. That hydrolysis prevails at pH >10 is supported by detailed experimental results (Figure SOTERM-6). These data are explained by the predominance in this system of Th(OH)(CO3)4 5- complex rather than Th(CO3)5 6- . A greater role for other ternary complexes of thorium (e.g., Th(OH)2(CO3)2 2-), which are also likely to be present in the WIPP conditions, is also proposed, and formation constants for these complexation reactions are reported. The use of the pentacarbonyl complex for the IV actinides in the WIPP PA, for these reasons, is a conservative assumption that overpredicts the solubility of the IV oxidation state at pH > 10. A correction in the FMT database, now in the EQ3/6 database, to the value of the Th(OH)4(aqueous [aq]) to be consistent with Neck et al. (Neck et al. 2002) was incorporated into the CRA-2004 PABC and there are no new changes in this speciation scheme in CRA-2014.
The dissolution of crystalline ThO2 in low ionic strength media and the effect of carbonate and calcium concentration on the solubility of thorium were investigated at alkaline pH (Kim et al. 2010). The observed thorium concentration in the groundwater was greater than predicted. This discrepancy was explained by the authors as the result of colloid formation. Carbonate affected the observed thorium solubility as expected. There was no calcium enhancement of the thorium solubility until a calcium concentration of 1.25 mM.
Oxyanions such as phosphate and, to a lesser extent, sulfate, also form Th4+ complexes that can precipitate at pH <5. The effect of phosphate on solubility of microcrystalline ThO2 is very limited. The stability constants for Th4+/H2PO4 - and Th4+/HPO4 2- were reported (Langmuir and Herman 1980). Overall, the role of these oxyanions is expected to be unimportant for the mildly basic brines (pH ~8-10) present in the WIPP.
A new perturbation to the understanding of Th speciation, as well as other actinides in the IV oxidation state, is the recent observation that Ca, and to a lesser extent, magnesium (Mg), enhances Th solubility at pH >10 when carbonate is present (see Figure SOTERM-7). In recent publications, the formation of Ca4[Th(OH)8]4+ and Ca4[Pu(OH)8]4+ ion pairs in alkaline CaCl2 solution is reported (Brendebach et al. 2007; Altmaier, Neck, and Fanghänel 2008). These species cause a rapid increase in the solubility of all tetravalent actinides at pH greater than 11. This increased solubility is only observed at CaCl2 concentrations above 0.5 M for Th(IV), and correspondingly above 2 M for Pu(IV) species. This effect can be discounted for the WIPP PA because Ca concentrations in the WIPP are predicted to be approximately 14 mM or less with a pH of approximately 8.7. These are both well below the levels needed to see a significant effect for both Th and Pu.
Actinides in the IV oxidation state, because of the complexity of their solution chemistry and very high tendency towards hydrolysis, form colloidal species in groundwater. The potential effect of colloid formation on solubility of Th(IV) in concentrated NaCl and MgCl2 solution was recently published by Altmaier, Neck, and Fanghänel (Altmaier, Neck, and Fanghänel 2004) and is shown in Figure SOTERM-8. In neutral-to-alkaline solutions, colloids could be formed as Th oxyhydroxide with log [Th](colloid [coll]) = -6.3 ± 0.5, independent of ionic strength. In Mg solutions, the formation of pseudocolloids (i.e., Th(IV))sorbed onto Mg2(OH)3Cl·4H2O(coll) led to an apparent increase of the total Th concentration up to 10-5 M (Walther 2003; Degueldre and Kline 2007; Bundschuh et al. 2000). For these reasons, colloid formation is addressed in the WIPP PA.
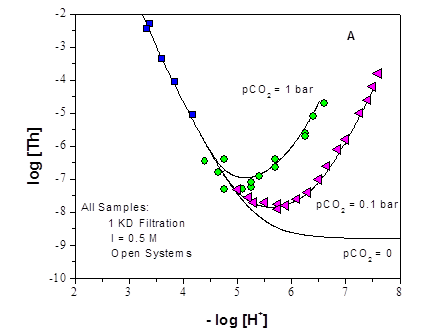
Figure SOTERM- 6. Solubility of Amorphous Th(IV) Oxyhydroxide as a Function of Carbonate Concentration in 0.5 M for (A) pH = 2-8 and (B) pH = 8-13.5. The solid lines are the calculated solubilities (based on data in Altmaier et al. 2005).
Figure SOTERM- 7. Effect of Calcium-carbonate Ternary Complexes on the Solubility of Th(IV) in Brine (Altmaier 2011).
Figure SOTERM- 8. Solubility of Th(OH)4(am) Determined from Undersaturation in 0.5 NaCl, 5.0 M NaCl, and 2.5 M MgCl2. Filled Points: Total Th Concentrations (Including Colloids); Open Points: Th Concentrations Measured after Ultracentrifugation at 90,000 Revolutions Per Minute (5 × 105 g) (based on data in Altmaier, Neck, and Fanghänel 2004).
A study to establish the solubility of thorium under WIPP-specific conditions was completed since CRA-2009. These experiments were performed in carbonate-free and carbonate-containing WIPP simulated brine to establish the effects of carbonate, pCH+ and time on thorium (IV) solubility and are published in a report entitled "Solubility of An(IV) in WIPP Brine, Thorium Analog Studies in WIPP Simulated Brine" (Borkowski et al. 2012).
The results obtained are shown in Figure SOTERM-9. After 2 years of equilibration in carbonate-free brine, the measured solubility of thorium was 6-7×10-7 M and was essentially independent of pH and brine composition over the 6.5 to 11.5 pCH+ range investigated. Sequential filtration to ~ 10 nm pore size had little effect on the measured concentration. Subsequent ultracentrifugation up to 1,000,000 g resulted in up to a 40% colloidal fraction (but typically 20% or less), indicating that there was much less intrinsic colloid formation than reported in Altmaier, Neck and Fanghänel (2004) - see Figure SOTERM-8. The steady-state thorium concentrations measured, however, are consistent with literature reports for simplified brine systems (Altmaier, Neck and Fanghänel 2004) but show a significantly lower extent of aggregation to form intrinsic colloids.
Figure SOTERM- 9. The Concentration of Thorium Measured in WIPP Simulated Brine (GWB and ERDA-6) as a Function of Time, Filtration and the Presence of Carbonate. Square symbols represent an undersaturation approach, whereas the circles represent the oversaturation approach. Although high, but metastable, concentrations were initially present, in time the measured concentrations decreased and are at or below the WIPP model-predicted values (Borkowski et al. 2012).
After an additional 2 years of equilibration, the thorium concentration in carbonate-free GWB significantly decreased (green points in the figure). For pCH+ in the range of 7.5 to 8.3 (not all data shown in Figure SOTERM-9), some samples did not show a change in the thorium concentration, but others showed a decrease of over one order of magnitude and were similar to the thorium concentrations measured in GWB containing 10-2 and 10-3 M carbonate.
The presence of carbonate, at a concentration that is ten-fold greater than expected in the WIPP, had little/no effect on the measured thorium concentrations. After two years of equilibration, the thorium concentrations measured from under- and oversaturation in GWB did not depend on carbonate concentration. Concentrations measured from oversaturation were 2.5 orders of magnitude greater than those measured from undersaturation, indicating that metastable states can persist for long periods of time. The trend in the oversaturation data (see Figure SOTERM-9) is consistent with the literature data (Altmaier et al. 2005). In the undersaturation experiments, which are more relevant to the WIPP situation, the average thorium concentration was 2×10-8 M and continued to decrease at pCH+ > 9. The oversaturation experiments showed a similar trend and at pCH+ > 9 the thorium concentrations decreased to below 10-8 M. These results reproduce, to some extent, the trends reported in the literature (Altmaier et al. 2005), but the much higher ionic strength solutions used in our experiments shift our pH profile to a lower pCH+ value by approximately 1 pH unit.
At the expected WIPP repository pCH+(~ 9.5), in the presence of carbonate, the thorium concentrations in GWB brine were 2×10-8 M or lower. This concentration trend suggests that at repository conditions the mixed thorium hydroxy-carbonato complexes do not play any role in the thorium solubility at pCH+ > 9.
The sequential filtration of thorium in the carbonate system (see Figure SOTERM-10) led to a dissolved thorium concentration of 2-6×10-8 M in GWB. In ERDA-6 brine, however, the dissolved thorium concentration was about ten-fold greater and it is apparent that steady state thorium concentration was not achieved. The colloidal thorium species appear to be very small, less than 10 kDa (~5 nm). Overall, the truly dissolved thorium concentration was 3(±2) × 10-8 M. The average total thorium concentration consisted of a dissolved fraction of 30 - 60% and a colloidal fraction of 40 - 70%.
The WIPP-specific thorium solubility results just summarized support the ongoing WIPP recertification effort in three important ways: 1) they provide empirical solubilities over a broad range of conditions that improve the robustness of the WIPP PA model, 2) they resolve and address published literature data in simplified brine systems that appeared to disagree with the current WIPP PA approach, and 3) they provide an input that will help establish the intrinsic colloidal enhancement factors for IV actinides. There is general agreement between our data and results reported in the literature for simplified brine systems, although we are seeing a far lower colloidal fraction in the total concentrations measured. After 4 years of equilibration, our measured solubilities are slightly lower (by a factor of ~ 2) than the solubilities calculated in the WIPP PA - this is well within the order of magnitude uncertainty typically observed between the calculated and measured solubilities in complex brine systems.
A key motivation in the WIPP thorium solubility and speciation studies was to explain the reports in the literature that very high colloidal fractions are present in high ionic-strength brine systems (mainly Altmaier et al. 2004). The WIPP-specific data show that there are colloids present in these systems, but these are much less than what was reported. The explanation for this is a combination of the differences in brine composition (sodium chloride brine vs. GWB/ERDA-6) between the two studies and the presence of MgO colloids in the Altmaier study where mineral fragment colloids were likely formed (which is counted as part of their colloidal fraction). Perhaps a more important result in the WIPP-specific studies is the observation that there is an equilibration between the intrinsic colloidal fraction and the dissolved species. This equilibrium shifts to a lower overall solubility with time that is now consistent with WIPP modeling predictions. This long-term shift defines these higher initial and essentially pH independent values for thorium solubility (Figure SOTERM-9 and SOTERM-8) that were obtained in both the German and WIPP data as metastable concentrations of thorium and explains the apparent discrepancy between model-predictions and experimental results. These solubility data support the current WIPP PA assumptions on An(IV) solubility and extend
past project data to a broader range of pH and carbonate levels. These results also note that Ca-enhanced hydroxyl complexation can greatly increase the solubility of actinides (IV), something that has only been understood in the last couple of years; however, this complexation requires relatively high pH in combination with very high Ca levels, something that is not expected in the WIPP. The expected pH and dissolved Ca levels in the WIPP predict no effect on An(IV) dissolved concentration due to formation of this complex.
Figure SOTERM- 10. Thorium Concentration in Simulated WIPP Brine as a Function of Pore Size. Ultrafilters used are given at the top of the figure and correlate with the filter pore size on the x axis. The % numbers shown correspond to the % of thorium that passed through the filter for each data point.
Uranium is not a TRU component but is, by mass, the predominant actinide in the WIPP. Current estimates predict that ~226 metric tons will be placed in the repository (Van Soest 2012), but this is believed to be a high estimate since uranium content in waste is often indirectly determined. By mass, approximately 85% of this will be the 238U isotope, with minor amounts of 233U, 234U, 235U, and 236U. Uranium does not contribute significantly to actinide release through cuttings/cavings and spallings because of its low specific activity (1.22×104Bq.g-1). Uranium release can occur through the Culebra in very small amounts because of its potentially high solubility and low partition coefficient (Kd) in the VI oxidation state.
Uranium release, as the 234 U isotope, was calculated in the CRA-2014 PA. In the WIPP PA, the oxidation state distribution assumption is that U speciates as U(IV) in the reduced PA vectors and as U(VI) in the oxidized vectors (Section SOTERM-4.1). The concentration for U(VI) is currently set at 1 mM (U.S. EPA 2005), since there is no An(VI) model in the WIPP. U(IV) solubility is calculated using the Th(IV) speciation data in the WIPP model.
Uranium is by far the most studied of the actinides under environmentally relevant conditions. An extensive review of this chemistry, as it relates to the WIPP case, was completed in 2009 (Lucchini et al 2010a; U.S DOE 2009), and is updated herein. More general reviews can be found (Morss, Edelstein, and Fuger 2006; Guillaumont et al. 2003; Runde and Neu 2010). An overview of U environmental chemistry is presented in this section.
Uranium can theoretically exist in aqueous solution in the III, IV, V, and VI oxidation states (Hobart 1990; Keller 1971 [pp. 195-215]; Clark, Hobart and Neu 1995). In the environment, however, only the IV and VI oxidation states, which exist as U4+ and UO2 2+ species, are present. U3+, should it be formed, is metastable and readily oxidized in aqueous solution, and U(V) only exists as a very short-lived transient that instantaneously disproportionates to form U(IV) and U(VI) species. The corresponding reduction potential diagram for U at pH = 0, 8, and 14 is given in Figure SOTERM-11 (Morss, Edelstein, and Fuger 2006).
Figure SOTERM- 11. Reduction Potential Diagram for U at pH = 0, 8, and 14 (Based on Data in Morss, Edelstein, and Fuger 2006). For the expected reducing and mildly basic pH conditions in the WIPP, U(IV) is predicted to be the predominant oxidation state.
Under oxidizing subsurface conditions typical of most near-surface groundwater, U(VI) as UO2 2+ uranyl complexes is the predominant oxidation state and is not easily reduced geochemically. Thermodynamically, uranyl species are stable even under mildly reducing conditions and are not reduced by some Fe(II) phases (see Table SOTERM-5). In anoxic WIPP brine experiments with a hydrogen overpressure, uranyl persists as a stable hydrolytic or carbonate complex for over two years (Reed and Wygmans 1997).
In the anoxic and strongly reducing environment expected in the WIPP, however, potential reduction pathways exist. The two most important of these reduction pathways are reaction of uranyl with reduced iron phases (Fe[0/II]), and bioreduction by anaerobic microorganisms (e.g., metal and sulfate reducers). For these reasons, U(IV) is the oxidation state expected to predominate in the WIPP when brine inundation occurs.
The use of iron barriers in the removal of uranyl from groundwater is well established and has been reported for the removal of U(VI) from groundwater using zero-valent iron barriers (Gu et al. 1998; Fiedor et al. 1998; Farrell et al. 1999) and iron corrosion products formed in saline solution (Grambow et al. 1996). However, in those studies, it was unclear whether the removal of uranyl (UO2 2+) resulted from reductive precipitation or from adsorption onto/incorporation into the iron corrosion products (Gu et al. 1998). In their experiments under saline conditions, Grambow et al. (Grambow et al. 1996) found that a large percentage of U was rapidly adsorbed onto the iron corrosion products consisting of over 97% hydrous Fe(II) oxide, and very little U(IV) was found. Recently, Myllykylä and Ollila (Myllykylä and Ollila 2011) observed the presence of U(IV) after adding an excess of Fe(II) to 0.01M NaCl and 0.002M NaHCO3 solutions containing U(VI) inside an anaerobic glovebox.
Under anoxic conditions, Trolard et al. (Trolard et al. 1997) established that the corrosion of steel and iron generates Fe(II)/Fe(III) hydroxide species known as green rusts. Green rusts contain a certain amount of nonhydroxyl anions (carbonate, halides, or sulfate); they have a high specific surface area (Cui and Spahiu 2002) and a high cation sequestration capacity (O'Loughlin et al. 2003). They are considered metastable oxidation products of Fe(II) to magnetite Fe3O4 and Fe(III) oxyhydroxides (e.g., goethite α-FeOOH) (O'Loughlin et al. 2003). They could be generated by iron corrosion in the WIPP brines (Wang et al. 2001). A few experimental studies demonstrate that U(VI) is reduced to U(IV) by green rusts (Dodge et al. 2002; O'Loughlin et al. 2003).
Recent studies suggest that magnetite stoichiometry can significantly influence the extent of U(VI) reduction (Latta et al. 2012). Latta et al. (Latta et al. 2012) demonstrated that stoichiometric and partially oxidized magnetite (Fe2+/Fe3+ ≥ 0.38) reduce U(VI) to U(IV) in UO2 nanoparticles in 2mM NaHCO3 solution at pH 7.2, whereas with more oxidized magnetite (Fe2+/Fe3+ < 0.38), possibly sorbed U(VI) is the dominant phase observed. Atomistic simulations conducted by Kerisit, Felmy and Ilton (Kerisit, Felmy and Ilton 2011), supported by existing Extended X-Ray Absorption Fine Structure (EXAFS) data provide strong evidence for the structural incorporation of U in Fe (hydro)oxides. The complexity of the U-Fe-H2O-CO2 system can explain the lack of a predominant mechanism (reduction-precipitation or adsorption/incorporation) for the removal of U(VI) in the presence of iron phases (Du et al. 2011; Ilton et al. 2012; Singer et al. 2012a; Singer et al. 2012b).
Banaszak, Rittmann, and Reed (Banaszak, Rittmann, and Reed 1998) have reviewed the important role of microbial processes in the reduction of multivalent metals under anaerobic/reducing conditions. For uranyl in particular, several studies exist that show that U(VI) is reduced to U(IV) species under a wide range of conditions (Lovley et al. 1991; Lovley et al. 1993; Barton et al. 1996; Huang et al. 1998; Abdelouas et al. 2000; Bender et al. 2000; Fredrickson et al. 2000; Suzuki et al. 2003). Most of this work pertains to groundwater bacteria, and is not directly applicable to the WIPP.
There are relatively few studies that investigate the interaction of U with the halophiles that are more typically present in the WIPP brine (Francis et al. 2004). Some WIPP-relevant research was done (Francis et al. 2000), but this work was mostly focused on gas generation, not actinide interactions. It remains to be demonstrated that the mechanisms leading to the bioreduction of U(VI) also extend to the microbes present in the WIPP.
Tetravalent U is expected to be the dominant oxidation state in the WIPP as a result of the reducing conditions that will prevail. The solubility of U(IV) under these conditions is analogous to that observed for Th (see Section SOTERM-3.3) and is, in fact, calculated in the WIPP PA with the Th(IV) database.
Experimentally, in solution, U4+ is readily oxidized to UO2 2+. This occurs even when only trace levels of oxygen exist that are often below the limit of detection by most laboratory instrumentation. This explains why there are relatively few studies of U4+. It is also problematic because there are very large discrepancies in the literature as a result of experimental artifact. In particular, there are a number of published results (Rai, Felmy, and Ryan 1990; Gayer and Leider 1957; Ryan and Rai 1983; Tremain et al. 1981; Casas et al. 1998) that suggest amphotericity for U4+ at pH >10. This, however, likely resulted from combined effects of two experimental artifacts: (1) oxidation to UO2 2+, which is much more soluble, and (2) the presence of carbonate, which is a strong complexant of U4+.
The solubility of U(IV) phases were also determined in simplified brines under conditions that relate to the WIPP (Rai et al. 1997; Rai et al. 1998; Yajima, Kawamura, and Ueta 1995; Torrero et al. 1994). These data are shown in Figure SOTERM-12. Rai et al. (Rai et al. 1997) determined the solubility of freshly precipitated UO2 ×xH2O(am) in NaCl and MgCl2 solutions of various ionic strengths. They estimate the concentration of U(OH)4(aq) in equilibrium with UO2 ×xH2O(am) to be about 10-8.0 M, and a number of data with greater concentrations in the neutral and alkaline range are ascribed to the presence of U(VI) in solution. This is in fair agreement with the value of 10-(8.7 ± 0.4) M proposed by Yajima, Kawamura, and Ueta (Yajima, Kawamura, and Ueta 1995). It is important to note that U(IV) concentrations at pH >5 show no significant dependence on the initial solid phase; both fresh precipitates in oversaturation experiments or electrodeposited microcrystalline UO2(s) in undersaturation experiments gave the same results (Torrero et al. 1994).
Figure SOTERM- 12. Solubility of UO2(s) as a Function of pH at 20-25 ºC (68-77 °F) in 1M NaCl (based on Neck and Kim 2001). The experimental data are from Ryan and Rai (1983), Rai et al. (1997), and Neck and Kim (2001). The solid line is calculated by Neck with Log Ksp = (-54.5 ± 1.0) and the hydrolysis constants selected in Neck and Kim (2001). The dotted lines show the range of uncertainty. The dashed line is calculated with the model proposed by Rai et al. (1997).
U(VI) phases and aqueous species, although not expected to predominate in the WIPP, could be present due to the localized effects of radiolysis (see Section SOTERM-2.4.2). The WIPP PA currently makes the conservative assumption that U(VI) species predominate in 50% of the PA vectors. The solubility of U(VI) is, however, not explicitly calculated in the WIPP PA, since there is no model for actinides in the VI oxidation state. The potential contribution of U(VI) species to the overall solubility of U in the WIPP is implicitly considered in the WIPP PA in the 1 mM value for U solubility (U.S. EPA 2005). Prior to this, the solubility of U was defined as 1.2 × 10-5 M based on an assessment of the literature and existing WIPP-relevant experimental data by Hobart and Moore (Hobart and Moore 1996).
The solubility of U(VI) in the WIPP is expected to be defined by the combined contribution of two processes: hydrolysis with oxyhydroxide phase formation, and carbonate complexation with U carbonate phase formation. These are both very complex systems, and there are many proposed speciation schemes. In carbonate-free or low-carbonate solutions, the speciation of U(VI) is dominated by hydrolysis.
Yamazaki et al. (Yamazaki et al. 1992) conducted U(VI) solubility experiments from both oversaturation and undersaturation in a synthetic brine at pCH+ values ranging from 6.4 to 12.4. The composition of this synthetic brine was close to the composition of the WIPP GWB brine, with higher concentrations of NaCl, NaBr, KCl and MgCl2 and ionic strength ~6 M. This synthetic brine initially contained 0.11 mM of bicarbonate HCO3 -, but the solution treatment (continuous nitrogen gas flow above the solution) likely removed some of the carbonate from solution before the later uranium additions and prevented any CO2 uptake during the experiment. The results obtained at the pCH+ closest to WIPP repository conditions with no further carbonate additions are listed in Table SOTERM-12. Uranium (VI) concentrations of approximately 10-7 M were observed at pCH+ = 10.4 and 12.4 when nitrogen gas was continuously passing over the solutions to minimize CO2 uptake. Despite extensive precipitation of brucite Mg(OH)2 at these high pCH+ values, the solubility-controlling phase at pCH+ ≥ 9.3 was found to be potassium diuranate K2U2O7.
Diaz-Arocas and Grambow (Diaz-Arocas and Grambow 1998) investigated uranium (VI) solubility in NaCl solutions up to 5 M at 25 °C and different basic pH values, under an argon atmosphere using an oversaturation approach. Their uranium concentration equilibria in 5 M NaCl are presented in Table SOTERM-12. At pH ≥ 7.5, poorly crystalline sodium-uranates, identified by XRD, were formed in solutions. Diaz-Arocas and Grambow indicated that the solubility of this phase was about 3×10-5 M at pCH+ = 8.9 in 5 M sodium chloride in the absence of carbonate.
Carbonate, as CO3
2-, has a significant effect on the solubility of U(VI) (Clark, Hobart and Neu 1995; Guillaumont et al. 2003). In the absence of competing complexing ligands, carbonate complexation will dominate the speciation of the uranyl ion under near-neutral pH conditions as long as there is ample carbonate-bicarbonate available (Clark, Hobart and Neu 1995). Complexation constants for binary U(VI) carbonate complexes at I = 0 M and 25 ºC (77 °F) are listed in Table SOTERM-12 (Guillaumont et al. 2003).
Table SOTERM- 12. Solubility of U(VI) in High-Ionic-Strength Media
|
U(VI) Concentration (M) |
pCH+ |
Solution |
Time (days) |
Solid |
Reference |
|
(2.8 ± 1.8) ×10-5 |
8.9 |
5M NaCl |
≈ 50 |
Na0.68UO3.34 × (2.15±0.10)H2O |
Diaz-Arocas and Grambow 1998 |
|
(8.2 ± 4.6) ×10-5 |
7.6 |
5M NaCl |
≈ 110 |
Na0.45UO3.23 × (4.5±0.1)H2O |
Diaz-Arocas and Grambow 1998 |
|
(4.2 ±1.9) ×10-4 |
7.1 |
5M NaCl |
≈ 170 |
Na0.29UO3.15 × (2.9±0.2)H2O |
Diaz-Arocas and Grambow 1998 |
|
(2.8 ± 0.9) ×10-6 |
6.5 |
5M NaCl |
≈ 170 |
Na0.14UO3.07 × (2.5±0.1)H2O |
Diaz-Arocas and Grambow 1998 |
|
(1.82 ± 0.01) ×10-3 |
8.4 |
Brine |
100 |
α-schoepite (oversaturation) |
Yamazaki et al. 1992 |
|
(1.81 ± 0.01) ×10-3 |
8.4 |
Brine |
100 |
α-schoepite (oversaturation) |
Yamazaki et al. 1992 |
|
(1.40 ± 0.05) ×10-3 |
8.4 |
Brine |
244 |
α-schoepite (undersaturation) |
Yamazaki et al. 1992 |
|
(1.80 ± 0.05) ×10-3 |
8.4 |
Brine |
244 |
α-schoepite (undersaturation) |
Yamazaki et al. 1992 |
|
(3.8 ± 0.4) ×10-7 |
10.4 |
Brine |
150 |
Mg(OH)2 and K2U2O7 (oversaturation) |
Yamazaki et al. 1992 |
|
(3.1 ± 0.3) ×10-7 |
10.4 |
Brine |
150 |
Mg(OH)2 and K2U2O7 (oversaturation) |
Yamazaki et al. 1992 |
|
(1.7 ±1.4) ×10-7 |
8.1 |
ERDA-6 |
705 |
To be determined (oversaturation) |
Lucchini et al. 2013b |
|
(9.9 ± 3.0) ×10-8 |
9.6 |
ERDA-6 |
705 |
To be determined (oversaturation) |
Lucchini et al. 2013b |
|
(3.1 ± 1.3) ×10-8 |
10.5 |
ERDA-6 |
705 |
To be determined (oversaturation) |
Lucchini et al. 2013b |
|
(2.1 ± 0.6) ×10-6 |
7.4 |
GWB |
705 |
To be determined (oversaturation) |
Lucchini et al. 2013b |
|
(4.3 ± 1.3) ×10-6 |
8.2 |
GWB |
705 |
To be determined (oversaturation) |
Lucchini et al. 2013b |
|
(8.1 ± 2.4) ×10-7 |
9.2 |
GWB |
705 |
To be determined (oversaturation) |
Lucchini et al. 2013b |
|
(2.7 ±0.5) ×10-7 |
8.0 |
ERDA-6 (initial 2mM carbonate) |
994 |
To be determined (oversaturation) |
Lucchini et al. 2013a |
|
(3.2 ± 1.0) ×10-5 |
8.8 |
ERDA-6 (initial 2mM carbonate) |
994 |
To be determined (oversaturation) |
Lucchini et al. 2013a |
|
(3.5 ± 2.8) ×10-8 |
12.1 |
ERDA-6 (initial 2mM carbonate) |
994 |
To be determined (oversaturation) |
Lucchini et al. 2013a |
|
(2.6 ± 0.8) ×10-6 |
7.6 |
GWB (initial 2mM carbonate) |
994 |
To be determined (oversaturation) |
Lucchini et al. 2013a |
|
(7.1 ± 1.4) ×10-7 |
9.0 |
GWB (initial 2mM carbonate) |
994 |
To be determined (oversaturation) |
Lucchini et al. 2013a |
Table SOTERM- 13. Complexation Constants for Binary U(VI) Carbonate Complexes at I = 0 M and 25 ºC (Guillaumont et al. 2003)
|
Reaction and Solubility Product for UO2CO3(crystalline [cr]) |
|
|
UO2CO3(cr) ⇌ UO2 2+ + CO3 2- |
Log K0 SP(cr)= -14.76 ± 0.02 |
|
Reactions and Formation Constants β0 nq for (UO2)n(CO3) q 2n-2q |
|
|
UO2 2+ + CO3 2- ⇌ UO2CO3(aq) |
Log β0 11 = 9.94 ± 0.03 |
|
UO2 2+ + 2 CO3 2- ⇌ UO2(CO3)2 2- |
Log β0 12 = 16.61 ± 0.09 |
|
UO2 2+ + 3 CO3 2- ⇌ UO2(CO3)3 4- |
Log β0 13 = 21.84 ± 0.04 |
|
3 UO2 2+ + 6 CO3 2- ⇌ (UO2)3(CO3)6 6- |
Log β0 36 = 55.6 ± 0.5 |
The three monomeric complexes of general formula UO2(CO3), UO2(CO3)2 2-, and UO2(CO3)3 4- are present under the appropriate conditions. There is also evidence from electrochemical, solubility, and spectroscopy data that support the existence of (UO2)3(CO3)6 6-, (UO2)2(CO3)(OH)3 -, and (UO2)11(CO3)6(OH)12 2- polynuclear species, which can only form under the conditions of high-metal-ion concentration or high ionic strength (Clark, Hobart and Neu 1995). At uranyl concentrations above 10-3 M, the trimeric cluster (UO2)3(CO3)6 6- can also be present in significant concentrations. When the uranyl ion concentration begins to exceed the carbonate concentration, hydrolysis will play an increasingly important role (Clark, Hobart and Neu 1995).
It is generally accepted that the major complex in solution at high carbonate concentrations is UO2(CO3)3 4- (Kramer-Schnabel et al. 1992; Pepper et al. 2004). However, at I = 0.5 M and I = 3 M, the polynuclear (UO2)3(CO3)6 6- species becomes an important competitor of UO2(CO3)3 4-. Grenthe et al. (Grenthe et al. 1984) indicated that the formation of (UO2)3(CO3)6 6- is favored at high ionic strengths as a result of possible stabilization of the complex by ions of the background electrolyte.
At high pH, Yamamura et al. (Yamamura et al. 1998) demonstrated that hydrolysis overwhelms carbonate complexation. The solubility of U(VI) was measured in highly basic solutions (11≤ pH ≤ 14) at an ionic strength of I = 0.5 - 2 M over a wide range of carbonate concentrations (10-3 - 0.5 M) using both oversaturation and undersaturation approaches. In the oversaturation experiments, the solubility of U(VI) decreased with increasing equilibration time from one week to one year and was explained as an increase in the crystallinity of the solid phase with aging. The solid phase was identified as Na2U2O7 ×xH2O by XRD. The undersaturation experiments conducted for one month with the solid phase indicated a rapid equilibrium. These data were interpreted by considering the formation of UO2(OH)3 -, UO2(OH)4 2- , and UO2(CO3)3 4- (Yamamura et al. 1998).
A few experimental investigations were reported on the influence of carbonate on U(VI) solubility in highly saline solutions (Yamazaki et al. 1992; Reed and Wygmans 1997; Lin et al. 1998; Fanghänel and Neck 2002). Lin et al. (Lin et al. 1998) evaluated U(VI) solubilities with up to 5M NaCl in a range of carbonate concentrations. At carbonate-ion concentrations greater than 10-7 M, UO2(CO3)3 4- was the dominant U(VI) complex in solution. At higher CO2 partial pressures, the solubility-controlling solid phase was found to be UO2CO3(s), whereas at lower partial pressures, sodium uranate was identified as the solid phase in NaCl-saturated solutions. This study, although interesting, is of questionable use to the WIPP because the details were not fully published.
Yamazaki et al. (Yamazaki et al. 1992) measured the solubility of U(VI) in synthetic brine and an air atmosphere. The results obtained at pCH+ = 8.4 using both oversaturation and undersaturation approaches are listed in Table SOTERM-12. At this pCH+ value, millimole concentrations of uranium were measured in solution. Solids obtained at pCH+ = 8.4 were identified as poorly crystalline schoepite (UO3·xH2O) by X-Ray Diffraction (XRD). Yamazaki carried out some calculations to model the competition between calcium and magnesium for carbonate complexation in order to interpret his experimental solubility data. He concluded that the uranium solubility decrease above pCH+ = 8.4 was related to a shift from the triscarbonato uranyl complex UO2(CO3)3 4- to the uranyl hydroxide complexes UO2(OH)n 2-n , as precipitation of calcium carbonate (CaCO3) occurred, and to the conversion of schoepite to potassium diuranate.
The only U(VI) solubility values available in the literature that were obtained in the presence of carbonate under WIPP-relevant conditions were featured in the fiscal year 1997 year-end report by Reed and Wygmans (Reed and Wygmans 1997). The experiments were carried out in ERDA-6 brine at pH 8 and 10, and in G-Seep brine at pH 5 and 7. U(VI), Np(VI), and Pu(VI) were added to the brine samples. CO3 2- (10-4 M) was also added to some of the samples. The experiments were conducted under a hydrogen atmosphere at 25 ± 5 °C. Concentrations and oxidation states of the actinides were monitored over time. The U(VI) concentration was stable at approximately 1×10-4 M when measured as a function of time in ERDA-6 brine at pH 10 in the presence of CO3 2- (Reed and Wygmans 1997).
The solubility of U(VI) in the absence and the presence of carbonate was extensively studied since the CRA-2009 in simulated GWB and ERDA-6 brine (Lucchini et al. 2010a, Lucchini et al. 2010b, 2013 and 2013a). A summary of these results is shown in Figure SOTERM-13 and a comparison of these results with other solubility data in the literature is given in Table SOTERM-12. No U(IV) solubility studies were conducted since Th(IV) is the analog for the IV actinides.
In the absence of carbonate, the measured U(VI) solubilities were about 10-6 M in GWB brine at pCH+ ≥ 7 and about 10-8 - 10-7 M in ERDA-6 at pCH+ ≥8 (Lucchini et al. 2007, 2010a and 2010b). These results put an upper bound of ~10-6 M for the solubility of uranyl in the carbonate-free WIPP brines for the investigated range of experimental conditions. At the expected pCH+ in the WIPP (~9.5), the measured uranium solubility was between 10-7 M and 10-6 M. In the presence of carbonate, the highest uranium solubility obtained experimentally was ~ 10-4 M, under WIPP-related conditions (pCH+ ~ 9.5). It is important to note that this uranium solubility, in the absence of carbonate, was 10-100 times lower than published results. The uranium (VI) solubility experiments reported in two other relevant publications (Yamazaki et al.1992; Diaz-Arocas and Grambow 1998) were performed in brines close to the WIPP brine composition, but possibly with a less rigorous control of a carbon dioxide-free environment. The impact of carbonate concentration on the solubility of uranium (VI) in the two simulated WIPP brines can be explained in terms of three distinctive pCH+ regions.
Figure SOTERM- 13. Uranium Concentration in ERDA-6 (Open Symbols) and GWB (filled symbols) versus pCH+. in Nitrogen Controlled Atmosphere, in the Absence of Carbonate or in the Presence of Two Concentrations of Carbonate (2×10-4 M and 2×10-3 M) at the Beginning of the Experiments. The carbonate systems data correspond to 17 samplings performed over 994 days.
The first pCH+ region is 7.5 ≤ pCH+ ≤ 8. In this pCH+ region, the uranium concentration was stable in both brines and independent of the carbonate concentration. However, there were small differences in the uranium solubility due to differences in the composition of the brine: ~ 10-6 M in GWB, and ~ 10-7 M in ERDA-6. These data indicated that there was no impact of carbonate in this pCH+ region (7.5 ≤ pCH+ ≤ 8), but there was certainly an effect due to one or more components of the brines that were present in higher amounts in GWB than in ERDA-6. Based on our investigation of neodymium solubility (Borkowski et al. 2010a), we postulated that borate may also play a role in defining the uranium (VI) solubility in this pCH+ region (see also Borkowski et al. 2010b). This possibility was confirmed experimentally (Lucchini, Borkowski and Richmann 2013; Lucchini et al. 2013a).
The second pCH+ region of interest, 8 ≤ pCH+ ≤ 10, is directly relevant to the WIPP. In this pCH+ region, not only was there a compositional effect between the two brines studied (higher uranium concentrations in GWB than in ERDA-6 for identical carbonate content), but there was also an impact of carbonate on the observed uranium solubility in each brine. At high carbonate content (2×10-3 M in our experiments), the uranium concentrations reached 10-4 M, which was two or more orders of magnitude higher than in the absence of carbonate. The low carbonate content data (2×10-4 M) did not reflect a strong influence of carbonate on uranium solubility, since the measured solubility was similar to the ones obtained in carbonate-free systems.
Lastly, the third pCH+ region of interest is at 10 ≤ pCH+. In that pCH+ region, the uranium concentrations were stable around 10-7-10-8 M. It is likely that hydrolysis overwhelmed any other possible effects on uranium solubility.
These newly obtained solubility data for uranium (VI) in the WIPP brine accomplished the following:
· Provided the first WIPP-relevant data for the VI actinide oxidation state that established the solubility of uranium (VI) over an extended pCH+ range for GWB and ERDA-6 brines in the absence or presence of carbonate
· Established an upper limit of ~ 10-6 M uranyl concentration at the reference pCH+ WIPP case in the absence of carbonate, and an upper limit of ~ 10-4 M uranyl concentration at the reference pCH+ WIPP case in the presence of 2 mM carbonate
· Confirmed a lack of significant amphotericity in the WIPP simulated brines at high pH values
· Demonstrated a small effect of borate complexation in the pCH+ range of 7.5 to 10
· Supported the current assumption in PA that the solubility of U(VI), under the expected range of conditions in the WIPP, will not exceed 1 mM
The WIPP repository is projected to contain ~32.5 kg of Np, primarily as the 237Np isotope (see Table SOTERM-8). Its inventory increases with time from the decay of 241Am and the possibility of 238U (n, 2n) reactions to 223 Kg at 1000y after emplacement. In the WIPP PA, Np speciates as Np(IV) in 50% of the PA vectors and as Np(V) in the other 50% of the PA vectors. The contribution of Np to actinide release from the WIPP was included in the CRA-2014 PA calculation, but its effect on release was negligible. Arguments have already been made that it should be excluded from consideration in the WIPP PA based on its low inventory (Brush and Garner 2005).
The environmental chemistry of Np is somewhat unique in the actinide series as a result of the relatively high stability of the NpO2 + species, which is in the V oxidation state, under a wide range of conditions typically found in the subsurface. This oxidation state is prevalent when oxidizing conditions predominate (Hobart 1990). It is mobile because it has a relatively high solubility and it is not strongly sorbed or complexed. It does not hydrolyze strongly, with little or no measurable hydrolysis until pH >9 (Neck, Kim, and Kanellakopulos 1992; Itagaki et al. 1992). Much of the complexation data for inorganic and organic complexes for Np pertains to the V oxidation state for this reason (Lemire et al. 2001). The log Ksp for NpO2OH (s) is 4.5 ± 0.06 (Neck, Kim, and Kanellakopulos 1992).
Np can, however, actually exist in up to five oxidation states in aqueous media. The redox potentials under basic conditions are (Martinot and Fuger 1985):
NpO5 3- → NpO2(OH)2 → NpO2OH → NpO2 → Np(OH)3 (SOTERM.28)
Only the Np(IV) and Np(VI) oxidation states, in addition to Np(V), can exist under the right conditions in reducing or oxidizing groundwater (Hobart 1990; Keller 1971 [pp. 195-215]; Clark, Hobart and Neu 1995). These exist as Np4+ complexes and NpO2 2+ complexes. Np(VI), unlike Np(V), is strongly hydrolyzed at near-neutral pH and is readily reduced by many constituents typically found in groundwater (e.g., organics and most reduced metals). For these reasons, it does not tend to persist in groundwater under most conditions.
Under reducing anoxic conditions, Np4+ species can predominate. These Np4+ species readily undergo hydrolysis and are comparable to Pu4+ in this regard. This system is highly irreversible and probably polymeric in nature, as is observed for Pu4+. The measured solubility of Np4+ is 10-8.5 to 10-8.1 M with Np(OH)4, not Np(OH)5 -, as the predominant aqueous species (Rai and Ryan 1985; Eriksen et al. 1993). The importance and predominance of the Np(IV) oxidation state in reducing conditions is even more pronounced when anaerobic bacteria are present. Np(V) was readily reduced by sulfate-reducing bacteria (Banaszak, Reed, and Rittmann 1998) and methanogenic consortia (Banaszak et al. 1999), and precipitated as Np(IV) solids.
In WIPP-specific experiments (Reed and Wygmans1997), spectroscopic evidence for the reduction of Np(VI) to Np(V) in ERDA-6 (Castile) brine at pH 10 was observed along with complete reduction of Np(VI) to Np(V) in G-Seep (Salado) brine at pH 7 when no iron or microbial activity were present. In the presence of oxalate, citrate, and EDTA, rapid and complete reduction of Np(VI) to Np(V) coupled with a slower formation of Np(IV) species was observed. The stability of Np(V) under these conditions is further confirmed by Neck, Runde, and Kim (Neck, Runde, and Kim 1995), who showed that Np(V) carbonate complexes are stable in 5M NaCl.
In the expected WIPP environment, however, where anoxic and reducing conditions with microbial activity and reduced iron are expected to be present, Np(IV) is expected to be the predominant oxidation state (Rai and Ryan 1985; Rai, Strickert, and McVay 1982; Kim et al. 1985; Pryke and Rees 1986). This is based on studies of the solubility of NpO2OH in 1 M and 5 M NaCl solutions at pH 6.5, where the reduction of Np(V) to Np(IV) was observed (Kim et al. 1985; Neck, Kim, and Kanellakopulos 1992).
There are no new WIPP-relevant results on the chemistry and speciation of Np since CRA-2009 and the CRA-2009 PABC. Neptunium is not a key contributor to release from the WIPP.
Plutonium is a key TRU component that contributes significantly to the potential for TRU release from the WIPP under all release mechanisms considered by PA. Pu isotopes, estimated to be ~12 metric tons at the time of closure, represent approximately 77% of the Ci content for actinides in TRU waste (see Table SOTERM-8) at emplacement. This changes with time to 62%, 83% and >99% at 100, 1000 and 10,000 years after emplacement due to radioactive decay and the relatively long half-life of 239Pu. There are five isotopes of Pu that make a significant contribution to the Pu inventory, but 239Pu, 238Pu, and 241Pu are the major contributors to the Ci content. Under the conditions expected in the WIPP, Pu(IV) is expected to be the predominant oxidation state (Weiner 1996). A more extensive review of Pu subsurface speciation issues as they pertain to the WIPP case was completed (Reed et al. 2009).
In the WIPP PA, all of the Pu is assumed to be reduced and present in the III or IV oxidation state. Half of the PA vectors contain 100% Pu(III), with the other half of the vectors containing 100% Pu(IV) species. Because the solubility of Pu(III) is roughly 10 times higher, the assumption that it is present is a conservatism built into the WIPP PA. The two higher-valent Pu oxidation states, Pu(V) and Pu(VI), are not considered in the PA because they cannot persist under the expected reducing and anoxic conditions in the WIPP.
Generally, Pu can exist in oxidation states III, IV, V, VI, and VII (Katz, Seaborg, and Morss 1986, p. 781). Of these, only Pu(V), Pu(IV), and Pu(III) are expected to be important under environmentally relevant oxidizing and reducing conditions. Pu(VII) is very unstable and exists only in extremely basic solutions (for example, 7 M NaOH) that are not expected in the WIPP. Pu(VI) and Pu(V) can persist in the WIPP in the absence of reductants, but they are readily reduced in the presence of Fe(II/0) species, reduced by many organic chelators (Reed et al. 1998), and possibly reduced in anaerobic, biologically active systems (Reed et al. 2007; Icopini, Boukhalfa, and Neu 2007). The reduction of Pu(VI/V), under WIPP-relevant conditions, was shown by Clark and Tait (Clark and Tait 1996), Reed and Wygmans (1997), and Reed et al. (Reed et al. 2007). In this context, only Pu(III) and Pu(IV) oxidation state species are expected to be present under WIPP-related conditions.
The role and importance of redox reactions in determining actinide mobility and solubility are beyond question (Van Luik et al. 1987; Allard 1982; Choppin and Rao 1992). The redox potentials for the various oxidation states at pH 7 are (Cleveland 1979, pp. 11-46)
A typical phase diagram for Pu in groundwater that illustrates the importance of redox is shown in Figure SOTERM-14.
Figure SOTERM- 14. Speciation Diagram for Plutonium in Carbonated Low-Ionic-Strength Groundwater (Based on Data Presented in Runde et al. 2002). This illustrates the expected lower solubility of reduced Pu(III) and Pu(IV) phases, and suggests that the dominant Pu species in the pH 8-9 range are hydrolytic species with lesser contributions from carbonate.
Higher-valent Pu, specifically Pu(V) and Pu(VI), can be present in near-surface oxidizing groundwaters (Orlandini, Penrose, and Nelson 1986). The association of Pu(V) with organic colloidal material was proposed as the mechanism by which subsurface migration occurred. Pu(VI), in near-neutral systems, is strongly and irreversibly hydrolyzed (Okajima and Reed 1993). It is also readily reduced by organics and reduced metal species even when oxygen is present to form Pu(V), and is not generally stable under most groundwater-relevant conditions.
Pu(V), by analogy with Np(V), does not undergo hydrolysis until pH >7 and tends to form weak complexes. It readily disproportionates to form Pu(IV) and Pu(VI) at high concentrations and is relatively easy to reduce in the environment under anoxic conditions. Fe2+(aq), Fe(II) minerals, and metallic iron reduce Pu(V) to Pu(IV).
In geochemical systems, redox control is often interpreted in terms of the iron, and in a broader sense, reduced metal, mineralogy, and associated aqueous chemistry (Sanchez, Murray, and Sibley 1985; White, Yee, and Flexser 1985). In the WIPP case, iron will undergo anoxic corrosion, producing Fe2+. Both metallic iron (Fe0) and Fe2+ have been shown to quantitatively reduce Pu(VI) in the WIPP brines to either Pu(IV) or Pu(III). Clark and Tait (Clark and Tait 1996) and Felmy et al. (Felmy et al. 1996) have experimentally observed the reduction of Pu(VI) carbonates by either Fe0 or Fe2+ to Pu(IV). In the absence of carbonates, a quantitative reduction of Pu(VI) is also observed, but the oxidation state of the resulting species cannot be definitively determined because its concentration is below the lower detection limit of the oxidation state analytical process (about 10-9 M). However, since this concentration is well below the expected solubility of Pu(V) species, it was reasonably assumed that the Pu must have been reduced to either the IV or III oxidation state. Neretnieks (Neretnieks 1982) has shown that when dissolved actinides in moving groundwater came in contact with Fe(II), the actinides were reduced to a much-less-soluble oxidation state and precipitated.
Pu(III) is not predicted to be stable under the expected WIPP conditions. There are, however, some mechanisms identified in which Pu(III) species can be formed. Felmy et al. (Felmy et al. 1989) observed some Pu(III) in the WIPP brines at neutral and slightly basic conditions. PA conservatively takes account of these minor mechanisms by assuming that Pu is speciated as Pu(III) in 50% of the PA vectors.
General studies of Pu in brine have been done by a number of investigators (Büppelmann et al. 1986; Büppelmann, Kim, and Lierse 1988; Clark, Hobart, and Neu 1995; Nitsche et al. 1992; Nitsche et al. 1994; Pashalidis et al. 1993; Villareal, Bergquist, and Leonard 2001; Reed et al. 1993; Reed, Okajima, and Richmann 1994; Reed and Wygmans 1997). There has also been an assessment of the actinide chemistry in the WIPP CCA (Oversby 2000; Brush, Moore, and Wall 2001; U.S. EPA 2006). These studies confirm reduction of higher-valent Pu under the expected WIPP conditions and establish the key speciation trends for Pu in the WIPP (see Figure SOTERM-15). These trends are captured in the WIPP PA through analogy with Am(III) for Pu(III) and with Th(IV) for Pu(IV).
Figure SOTERM- 15. The Concentration of Pu as a Function of Time in the Presence of Iron Powder, Iron Coupon, Ferric Oxide, and Magnetite (Mixed Iron Oxide) (Reed et al. 2009)
Comprehensive and critical reviews of how actinide species and microorganisms interact have been published (Banaszak, Rittmann, and Reed 1998; Neu, Ruggiero, and Francis 2002; Reed et al. 2010). The likelihood that this could occur and recent results with Fe(III) reduction were discussed in Section SOTERM-2.4.1.4. Additionally, the important role of microbial activity through biotic transformations (Zitomer and Speece 1993; Banaszak, Rittmann, and Reed 1998; Rittmann, Banaszak, and Reed 2002; Reed et al. 2007) in defining oxidation state distribution of multivalent metals and actinides has been recognized.
Although the bioreduction of uranyl and neptunyl species is well established, there are relatively few studies of the bioreduction of plutonyl species. Reed et al. (Reed et al. 2007) demonstrate that Shewanella alga, a ubiquitous metal-reducing soil bacterium, reduces Pu(V) to Pu(III/IV) species. Icopini, Boukhalfa, and Neu (Icopini, Boukhalfa, and Neu 2007) have shown that Geobacter and Shewanella oneidensis also reduce higher-valent Pu to Pu(III/IV) species.
These Pu data are consistent with the oxidation state predictions in microbially active systems. It is particularly important to note that Pu(IV) is the expected oxidation state under a wide range of anoxic subsurface conditions, with no Pu(V) or Pu(VI) species expected. The recent Pu bioreduction results confirm that highly reducing conditions are being generated by metal-reducing bacteria under anaerobic growth conditions and support the current WIPP PA assumption that higher-valent actinides cannot persist when the concentration of dissolved actinides is important and microbial activity is prevalent.
There are no studies on the bioreduction of Pu(V/VI) under WIPP-relevant conditions (note discussion in Section SOTERM-2.4.1.4. Halophilic microorganisms (Gillow et al. 2000; Swanson and Simmons 2013; Swanson et al. 2012) typically found and expected to predominate in the WIPP environment have not been studied for their ability to reduce higher-valent actinides, although they will contribute to the establishment of reducing conditions in the WIPP.
It has long been held that Pu oxide, as PuO2, is the thermodynamically favored form of Pu oxide. This oxide is likely the predominant form of Pu in TRU waste and is believed to be the most important phase under WIPP-related conditions. In the last few years, however, there have been a number of studies that question this key and fundamental assumption.
Haschke, Allen, and Morales (Haschke, Allen, and Morales 2000) report that near-stoichiometric plutonium dioxide reacts with water vapor at temperatures between 25 °C and 350 °C (77 °F and 662 °F) according to the following reaction:
PuO2(s) + xH2O(g) ® PuO2+x(s) + xH2(g) (SOTERM.30)
Here, water vapor is reduced by polycrystalline PuO2 to produce hydrogen (H) and a previously unknown higher-oxide PuO2+x with x as large as 0.27. If only Pu(IV) and Pu(V) are present in PuO2.27, this oxide has 46% Pu(IV) and 54% Pu(V). Once formed, the PuO2+x may dissolve in contact with groundwater to form aqueous PuO2 + or PuO2 2+ species (Haschke and Ricketts 1995).
There remains some controversy about the mechanisms that led to the observation of higher-valent Pu in the PuO2+x. This process only occurs under unsaturated conditions at high relative humidities. Haschke, Allen, and Morales (Haschke, Allen, and Morales 2000) argue that this conversion is due to a chemical reaction (that is, the above reaction has a Gibbs energy less than zero) rather than a radiolysis-induced reaction because the reaction rate is temperature dependent. However, there seems to be some contribution from radiolysis in this process and this may be the dominant mechanism (LaVerne and Tandon 2002). Neither of these mechanisms are expected to impact WIPP repository performance.
The behavior of PuO2 in contact with water was studied as a function of time by means of the short-lived isotope 238Pu, as well as the longer-lived 239Pu (Rai and Ryan 1982). This study concluded that crystalline PuO2, amorphous PuO2, and amorphous PuO3 ×xH2O all convert to a material intermediate between crystalline PuO2 and a hydrated amorphous material that contains both Pu(IV) and Pu(VI). These authors hypothesized that alpha particles generated by 238Pu or 239Pu irradiated water to generate OH radicals that reacted to form Pu(V) and/or Pu(VI) on the oxide surface. These observations are why the formation of localized oxidizing zones, where some higher-valent Pu can persist, is recognized in the WIPP PA. Reduction of these species, however, leads to a reformation of Pu(IV) hydrous oxide precipitates.
The overall issue of a thermodynamic driver for higher-valent Pu oxides, although it has received much recent attention in the literature, is not yet resolved, but has a relatively insignificant impact on the WIPP regardless of the mechanisms at work. A prolonged unsaturated phase in the WIPP could lead to the formation of some PuO2+x, but this will be quickly overwhelmed in an aqueous environment and the higher-valent Pu will be reduced to Pu(III/IV) species, as described in Section SOTERM-3.5.1.1 and Section SOTERM-3.5.1.2. Both DBR and transport-release scenarios assume brine inundation and, correspondingly, the rapid introduction of reducing conditions.
Since the CRA-2009 and CRA-2009 PABC, the WIPP-specific Pu-Fe interaction studies (Reed et al. 2010) were extended in time to almost 6 years to establish the long-term oxidation state distribution of plutonium in these iron-dominated brine systems. In these investigations 242Pu, initially as PuO2 2+, was used to minimize radiolytic effects. Additionally these were done in two WIPP-relevant brines (see Table SOTERM-4): GWB as a high magnesium brine typical of MgO-reacted brine, and ERDA-6 as a high sodium chloride brine typical of brine found in the far field. The initial oxidation state was established using absorption spectrometry (Varian CARY 5000) and solids were prepared from these brines using established methods. Initially, only Pu(IV) was evident in the XANES analysis (see Figure SOTERM-16). This correlated with a plutonium concentration that was in the range of 2 x 10-9 M to as high as 1.5 x 10-7 M at the lower end of the pH range (pH = 7). These data agreed with the results obtained in a prior study after approximately two years when Pu-239 was the plutonium isotope (Reed et al. 2007). After ~5.8 years, these same solid samples were re-analyzed and found to be mostly Pu(III) with a small amount of Pu(IV) in a few samples (see Figure SOTERM-17 and Table SOTERM-14). The observation of Pu(III) in the solid phase correlated with an increase in the plutonium solution concentrations from 1 x 10-8 M to 3x10-7 M (see Figure SOTERM-18). This is a slight elevation in concentration, by a factor of ~ 2 to 5, when compared to the earlier Pu(IV)-relevant data. This increased solubility is also consistent with the phase transformation to Pu(III) since the solubility of Pu(III) is expected to be somewhat higher than Pu(IV).
The plutonium (III/IV) solids data show a qualitative correlation with the Fe(II)/Fe(III) ratio and measured redox potential (Eh). Experiments with less negative Eh also had a greater amount of Fe(III) and Pu(IV) species present in the system. This adds to the linkages seen by others between the iron and plutonium chemistry in subsurface conditions. Although these specific experiments were performed in brine, they are consistent with the correlation between iron chemistry and other metals observed in low ionic strength groundwater (Masue-Slowey et al. 2011; Holm and Curtiss 1989; Christensen et al. 2000). The overall reaction sequence is given by:
The predominance of Pu(III) at long times provides a strong data point on the reducing conditions that iron creates under WIPP-relevant conditions, but does not account for radiolytic impacts on Eh, and the effects of organic complexation which will stabilize Pu(IV) relative to Pu(III). These data, taken in context, strongly support the current WIPP PA assumption that Pu(III) and Pu(IV) will be prevalent in the WIPP and both oxidation states will contribute to the actinide source term.
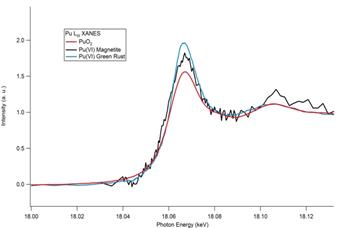
Figure SOTERM- 16. XANES Analysis of Plutonium Precipitates in the Magnetite and Iron Reduction Experiments at 3 Months. Pu(IV) phases were predominantly noted.
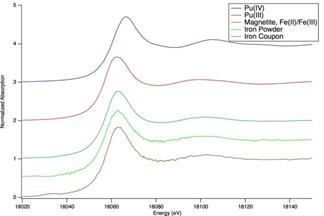
Figure SOTERM- 17. XANES Analysis of Solid Samples from the Pu-Fe Interactions Studies after ~ 6 Years. Pu(III) was the predominant oxidation state noted.
Figure SOTERM- 18. Effect of Filtration on the Measured Concentration of Plutonium as a Function of pCH+. Data shown are 0.45 µ (black squares), 0.22µ (green circles), 20 nm (blue diamonds) and 10 nm (red circles) filtrations. Uncertainty in the filtration data, based on ICP-MS analyses, is estimated to be ± 20%. The concentration of 10 nm-filtered plutonium at pCH+ ~ 9.5 is 3 x 10-7 M.
Table SOTERM- 14. Qualitative Redox Indicators for Iron Interactions with Plutonium under Anoxic Conditions
|
Experiment |
Description |
a Oxidation State of Pu Solid |
b [Fe]total in mM (%Fe2+ in solution) |
c Eh Measured (± 3 mV) |
|
PuFe23OX |
ERDA-6 brine at pH ~9 with excess magnetite |
~87% Pu(III), rest Pu(IV) |
0.12 (25%) |
-122 mV |
|
PuFeCE8 |
ERDA-6 brine at pH ~8 with Fe coupon |
~100% Pu(III) |
ND |
ND |
|
PuFeCE10 |
ERDA-6 brine at pH ~ 9.6 with Fe coupon |
~100% Pu(III) |
0.27 (100%) |
ND |
|
PuFeP |
ERDA-6 brine at pH~9 with excess Fe powder |
~100% Pu(III) |
0.18 (100%) |
-175 mV |
|
PuFeC |
ERDA-6 brine at pH ~ 9 with Fe coupon |
~90% Pu(III), rest Pu(IV) |
0.18 (58%) |
-110 mV |
|
PuFeG7 |
GWB brine at pH ~6.7 with Fe coupon |
~ 100% Pu(III) |
12.62 (97%) |
-210 mV |
|
a.Pu(III) content established by XANES analysis of solids b. Fe(II) content established by analysis using FerroZene® c.Eh measurement made using an Orion combination ORP electrode ND - not determined |
||||
There are relatively small quantities of Am in TRU waste (see Table SOTERM-9), and this is anticipated to be ~ 0.203 metic tons at emplacement. The high activity of 241Am (t½ = 432 years, 3.443 Ci/g) makes Am a key contributor to potential actinide release from the WIPP at earlier times in repository history (~26% initially, decreasing to 17% and ~0% at 1000 and 10,000 years after emplacement). In the WIPP PA, Am is in the trivalent state in all vectors and the aqueous concentration consists of Am3+ complexes and colloidal species.
Cm is also present in very small quantities in the WIPP (Table SOTERM-9) and exists primarily as the 244Cm isotope. The high activity of this isotope (t½ = 18.11 years) makes Cm an important species in the WIPP at the very early stages of repository history. It is essentially unimportant for the PA because it has decayed away by the end of the 100-year period for active institutional controls. However, other Cm isotopes with longer half-lives are present in the inventory and are considered by the WIPP PA. The environmental chemistry of Am and Cm are very similar, and most of what is said in this section about the environmental chemistry of Am also applies to Cm.
A more detailed review of the literature for Am can be found as part of a WIPP report (Borkowski et al. 2008). The solubility of An(III) was measured in the WIPP brine over a wide range of conditions using Nd(III) as a redox-invariant analog. These data support current WIPP PA calculations for the solubility of Pu(III) and Am(III) in the WIPP brine and are also summarized in Borkowski et al. (Borkowski et al. 2008).
Am is a 5f electron element and, like other elements of the actinide group, can exist in aqueous solution in several oxidation states. The electrode potentials for some Am couples are presented in Figure SOTERM-19. The trivalent state of Am is the most stable aqueous oxidation state (Katz, Seaborg, and Morss 1986, p. 912), and it is quite difficult to oxidize in aqueous solution (Hobart, Samhoun, and Peterson 1982). The trivalent Am ion has an ionic radius of 97.5 picometers (pm) (coordination number [CN]=6) and its chemical properties can be used as an analog for Pu(III), which has a similar ionic radius (100 pm at CN=6) and charge density, as well as for Cm(III) (97 pm at CN=6).
Figure SOTERM-
19. Redox Potential for Some Am Redox Couples (Silva et al. 1995,
p. 74)
The Am(II) species is italicized to stress that it is only a transient species. As discussed by Martinot and Fuger (Martinot and Fuger 1985), there is evidence for the formation of Am(II) in aqueous perchlorate solution in the pulse radiolysis experiment. The half-life of this species was estimated to be approximately 5 μs. This species is not observed during the electroreduction of Am(III) to the metal in noncomplexing media (David, Maslennikov, and Peretrukhin 1990).
Cm is also distinguished by the relatively great stability of the III oxidation state with respect to oxidation or reduction (Katz, Seaborg, and Morss 1986, p. 970). The stability of Cm(III) may be attributed to the half-filled f-shell electronic configuration (5f7). The oxidation of Cm(III) is achieved only with the strongest oxidizing agents, and only one report claims evidence for an oxidation state higher than IV (Korpusov, Patrusheva, and Dolidze 1975). The Cm(III) to Cm(IV) transition has not been successfully induced by ozone or electrochemically, and the Cm(IV) phosphotungstate produced by oxidizing with peroxysulfate is considerably less stable than the Am(IV) analog (Katz, Seaborg, and Morss 1986, p. 971). In the reducing environment of the WIPP repository, any higher-valent Cm produced radiolytically would be unstable. For all these reasons, the predominant oxidation state for Cm in the WIPP environment is Cm(III).
Higher-valent Am species have also been noted. Am(IV) species, with an ionic radius estimated by Shannon (Shannon 1976) to be 85 pm, is only stable in the presence of strongly complexing anions such as carbonate, fluoride, phosphate, or phosphotungstate, and was never found in any appreciable amount in trivalent Am solutions.
The pentavalent and hexavalent dioxoamericium ions AmO2 + and AmO2 2+ can be generated under strongly oxidizing conditions. Free radicals produced from α particles in water readily reduce these dioxoamericium ions back to Am3+. In concentrated NaCl solution, in which the radiolysis products are strong oxidants, pentavalent and hexavalent Am are the predominant species (Büppelmann et al. 1986). Without an oxidant, the pentavalent dioxoamericium ion slowly disproportionates to AmO2 2+ and Am3+. These higher oxidation states are not stable in natural waters and can be readily reduced by action of reductants naturally present in those waters.
The speciation of Am in groundwater under mildly alkaline conditions is primarily defined by hydrolysis and carbonate complexation. Hydrolysis is generally represented by the following reaction:
Am3+ + nH2O D Am(OH)n (3-n) + nH+ (SOTERM.32)
Silva measured the 243Am(OH)3(crystalline [cr]) and Nd(OH)3(cr) solubilities in 0.1 M NaClO4 solution at 25 ± 1 oC within the pH range 6 to 10 (Silva et al. 1995, p. 79-97). This is the only study with Am hydroxide using an x-ray-characterized crystalline solid. The solid phase was prepared by rigorously controlled, high-temperature transformation of Am(OH)3(am). Optical viewing by SEM of the solid samples at the end of the solubility experiments showed no changes in the crystal. The use of the 243Am isotope diminished α-particle damage of the crystal as a result of the 17-times-lower specific activity compared to 241Am. The weakness of this experiment was the relatively short equilibration time of only 48 days. A log (Ksp) of 16.6 ± 0.4 was obtained for the Am(OH)3 phase. The corresponding hydrolysis constants are listed in Table SOTERM-15. Similar values for Nd(III) hydrolysis were derived from the Nd(OH)3(cr) solubility measurements.
Stadler and Kim (Stadler and Kim 1988) investigate the pH dependence of Am(OH)3(s) solubility in 0.1 M NaClO4 and more concentrated Na chloride and perchlorate solutions at 25 ± 0.5 oC. The effect of α-induced radiolysis on solubility was also studied using different total concentrations of 241Am. The solid phase was not characterized in this work. Although the solid used in this work was different than that used by Silva et al. (Silva et al. 1995, pp. 275-76), the reported solubility products are in agreement. It is unclear, however, if the same phase controls the Am solubility in these two cases, because of markedly different preparation conditions of the starting solids.
Kim et al. (Kim et al. 1984) measured the solubility of Am(OH)3(s) at I = 0.1 and 0.3 M NaClO4, in the absence of CO2 and at pCO2 =10-3.5 atm, and attributed the solubility measured in terms of contributions from the hydroxy, carbonato- and mixed Am hydroxy-carbonato complexes. No characterization of the solid was reported in this work, so it was assumed to be AmCO3OH(s). Several investigators found that changes in the solid phase in aqueous suspensions of Am(III) hydroxide due to aging conditions become evident in hours and continue for weeks. Similar results were reported by Felmy, Rai, and Fulton (Felmy, Rai, and Fulton 1990). These authors measured the solubility of AmCO3OH(cr) at pCO2 =10-3 atm. The change in total Am concentration measured in this work as a function of pH was similar to that reported by Kim et al. (Kim et al. 1984). Similar plots for the solubility of Nd in 5 M NaCl were measured by Borkowski et al. (Borkowski et al. 2008); however, the Nd concentrations obtained for the comparable pCH+ values were two to three orders of magnitude greater as a result of the higher ionic strength present.
Table SOTERM- 15. Hydrolysis Constants of Am(III) (in Logarithmic Units) Corresponding to Equation SOTERM.32
|
AmOH2+ |
Am(OH)2 + |
Am(OH)3(aq) |
Medium |
Reference |
|
-7.93 ± 0.35 |
-14.77 ± 0.25 |
-24.71 ± 0.11 |
0.1 M NaClO4 |
Kim et al. 1984 |
|
-7.5 ± 0.3 |
-15.4 ± 0.4 |
-26.9 ± 0.5 |
0.1 M NaClO4 |
Stadler and Kim 1988 |
|
-7.8 ± 0.4 |
-15.4 ± 0.5 |
-26.9 ± 0.5 |
0.1 M NaCl |
Stadler and Kim 1988 |
|
-8.1 ± 0.3 |
-15.8 ± 0.4 |
-27.0 ± 0.5 |
0.6 M NaCl |
Stadler and Kim 1988 |
|
-7.7 ± 0.3 |
-16.7 ± 0.7 |
-25.0 ± 0.3 |
0.1 M NaClO4 |
Silva et al. 1995, p. 81 |
|
-6.9 ± 0.2 |
|
-23.8 ± 0.9 |
0.1 M NaClO4 |
Rösch et al. 1989 |
|
<-8.2 |
-17.1 ± 0.7 |
<-27.0 |
I → 0 |
Rai et al. 1983 |
|
-6.40 ± 0.11 |
-13.40 ± 0.16 |
-20.31 ± 0.17 |
3 M NaClO4 |
Pazukhin and Kochergin 1989 |
|
-7.0 ± 0.4 |
-15.1 ± 0.4 |
-26.4 ± 0.5 |
0.1 M NaClO4 |
Silva et al. 1995, p. 294 |
|
-7.2 ± 0.5 |
-15.1 ± 0.7 |
-26.2 ± 0.5 |
I = 0.1 M |
Neck et al. 2009, p. 1557 |
Am complexation by carbonate was extensively investigated by solvent extraction, spectrophotometry, electromigration, and solubility (Kim et al. 1984; Rösch et al. 1989; Felmy, Rai, and Fulton 1990; Meinrath and Kim 1991; Nitsche et al. 1995; Torretto et al. 1995). Many different soluble species have been proposed for the Am-water-carbonate system: pure carbonate, bicarbonate, and/or mixed hydroxy-carbonate complexes. Silva et al. (Silva et al. 1995) carefully studied and reinterpreted the literature data. It is the consensus in these studies that Am(CO3)n (3-2n), with n = 1, 2 and 3, are the predominant carbonate complexes. According to Silva et al. (Silva et al. 1995), there is no experimental evidence for the existence of a complex with n = 4 even at the highest carbonate concentrations. The report also suggests that there is no evidence for the formation of Am(III)-bicarbonate or hydroxy-carbonate complexes in solution. These data are, however, in disagreement with the more recent work done by Fanghänel and Kim (Fanghänel and Kim 1998), which reports spectroscopic evidence for the formation of the n = 4 species.
Data reported by Kim et al. (Kim et al. 1984) indicate that up to pCH+ = ~8.0, the carbonate complexation does not affect the solubility of Am(III). Analysis of Yuci groundwaters by Chen et al. (Chen et al. 2010), with a composition and Eh intermediate to the Yucca Mountain J-13 and UE-25 well compositions, demonstrates an americium carbonate solubility of 1.8x10-9 M at pH = 7.0 and 1.2x10-9 M at pH = 8.5 when equilibrated against solid AmOH(CO)3. The presence of 10-4- 10-2 M carbonate was shown not to influence amercium solubility in the pH range of 8-10. For the higher pCH+, the presence of carbonate in 0.1-0.3 M NaClO4 increases solubility of Am(III) in relation to carbonate-free systems, and at pCH+ = 10 this difference is almost 4 orders of magnitude. The predominance of carbonate complexation is observed in the pCH+ range from 7.5 to 10. At higher pCH+, hydrolysis predominates over carbonate complexation.
Neck et al. (Neck et al. 2009) used known data on the solubility of Am(OH)3, the hydrolysis of Am(III) and Cm(III), additional data from an extensive solubility study of Nd(OH)3(s) in NaCl, MgCl2 and CaCl2 media of various ionic strength media and time resolved laser induced fluorescence (TR-LIF) data for Cm(III) in alkaline CaCl2 to evaluate a comprehensive set of standard-state equilibrium constants and ion interaction parameters for the specific ion interaction theory SIT and Pitzer equations at 25 oC in the M(III) - H+ - Na+ - Mg2+ - Ca2+ - Cl- - OH- - H2O system. The solubility and hydrolysis behavior of Am(III), Cm(III) and Nd(III) in both calcium-free and calcium-containing solutions is consistently described using a model that includes the ternary Ca-M(III)-OH complexes Ca[M(OH)3]2+, Ca2[M(OH)4]3+ and Ca3[M(OH)6]3+. Data are presented in Neck Tables 1, 2 and 3 for the SIT and Pitzer parameters for this system. Solubility studies in NaCl - NaOH, NaClO4 - NaOH, pure NaOH and KOH solutions up to pH = 14 showed no evidence for the formation of Am(OH)4 -, which would increase the americium solubility at high pH. Study of the TR-LIF behavior of curium in alkaline solutions of various media at pH > 10 showed that Cm(OH)3(aq), which would be expected to dominate the speciation at pH = 11-14, nor the complex Cm(OH)4 -, could be detected, primarily due to low curium solubility. Almost all of the curium is present as Cmm(OH)3m polymers or colloidal Cm(OH)3(am). In alkaline CaCl2 solutions at I = 0.1 - 3M and pH ~ 10.5, as opposed to the sodium-based media above, the behavior of curium is strikingly different. Cm(III) emission bands were observed caused by complexes with three, four and six OH- ligands. These complexes, not found in NaCl - NaOH media, are stabilized by the association of Ca2+ ions, e.g., the ternary complexes Cap[Cm(OH)n]3+2p-n. Stability constants for the complexation reaction:
pCa2+ + Cm3+ + nH2O = Cap[Cm(OH)n]3+2p-n + nH+ (SOTERM.33)
are log *βo 1,1,3 = -26.3 ± 0.5, log *βo 2,1,4 = -37.2 ± 0.6 and log *βo 3,1,6 = -60.7 ± 0.5. These reactions do not affect the WIPP case under current conditions.
An extensive series of experiments, reported for CRA-2009, were performed to determine the solubility of Nd(III) as an analog for Pu(III) and Am(III) solubility in the brine (Borkowski et al. 2008). In this study, the solubility was determined in GWB and ERDA-6 brine, over a pH range of 6-12, and as a function of carbonate concentration. These solubility data extended earlier studies in simplified brines to simulated WIPP brine compositions and cover a broader range of experimental conditions. A composite of literature and WIPP-specific data is shown in Figure SOTERM-20.
Figure SOTERM- 20. Composite of Nd Solubility Trends Under All Conditions Investigated (Borkowski et al. 2008). Open symbols correspond to undersaturation experiments and closed symbols correspond to oversaturation experiments.
There are no new WIPP-specific data since CRA-2009 and CRA-2009 PABC that is centered on the solubility of An(III) in brine. New data showing the impacts of organic complexation are summarized in section 3.8.
The complexation of chelating agents with actinides has a significant impact on the concentrations of actinides in brine. At the pH of interest to the WIPP PA, only EDTA and citrate complex strongly enough to impact observed concentrations and this impact is mostly centered on the An(III) oxidation state.
The stability constants for organic ligand-actinide complexation were determined as part of the WIPP ASTP at Florida State University (Choppin et al. 1999). These data are summarized in Table SOTERM-16 and demonstrate some key trends in actinide complexation. For acetate, oxalate, and citrate, the strength of the complex formed is in the same order: IV > VI > III > V. For EDTA, the VI and III are switched. For the most part, the III and IV actinides, which are the two most important oxidation states in the WIPP, are strongly affected by organic complexation and thus can out-compete carbonate and hydrolysis if the organic concentrations are high enough. Of the four organic chelating agents considered, only citrate and EDTA are expected to form strong enough complexes to influence the speciation of actinides and potentially increase actinide concentrations under the expected conditions in the WIPP.
Table SOTERM- 16. Apparent Stability Constants for the Complexation of Organic Ligands with Actinides in NaCl Media (Choppin et al. 1999)
|
Organic Ligand |
Actinide Ion |
NaCl (molality) |
log10 β1 |
|
Acetate |
Am3+ Th4+ NpO2 + UO2 2+ |
0.3 to 5 0.3 to 5 0.3 to 5 0.3 to 4 |
1.44 - 2.2 3.68 - 4.18 1.05 - 1.8 2.23 - 3.09 |
|
Oxalate |
Am3+ Th4+ NpO2 + UO2 2+ |
0.3 to 5 0.3 to 5 1.0 to 5.0 0.3 to 5 |
4.17 - 4.63 7.04 - 7.47 3.62 - 4.63 5.82 - 6.7 |
|
Citrate |
Am3+ Th4+ NpO2 + UO2 2+ |
0.3 to 5 0.1 to 5 0.1 to 5 0.3 to 5 |
4.84 - 5.9 9.31 - 10.18 2.39 - 2.56 7.07 - 7.32 |
|
EDTA |
Am3+ Th4+ NpO2 + UO2 2+ |
0.3 to 5 0.3 to 5 0.3 to 5 0.3 to 4 |
13.76 - 15.1 15.56 - 16.94 5.45 - 6.7 10.75 - 12.16 |
The possible impact of isosaccharinic acid (ISA) on thorium speciation was also considered. ISA is a chemical breakdown product of cellulosic material that has been shown to occur at pH>12. The two diastereoisomers, a- and b-isosaccharinic acids, are the products of chemical degradation of cellulosic materials in alkaline solutions. The alkaline degradation of different cellulosic materials was studied for the alkaline conditions that may exist in the initial stages of a cementitious repository (pH ~ 13.3). ISA is expected to be present in cement pore water, but it is strongly adsorbed to the cement surface. In the pore water, the concentration of ISA is expected to reach 10-4 M (Van Loon et al. 1997). The complexation data for ISA is very limited and there are no literature references for the tetravalent cations such as thorium. ISA is structurally a 2-hydroxycarboxylic acid; therefore, by analogy we can relate the complexation of thorium by ISA to the stability constants for Th(IV) with glycolic acid, lactic acid and 2-hydroxybutanoic acid (log K of 4.3, 4.2 and 3.8 respectively). Ligands with such low stability constants cannot outcompete An(IV) hydrolysis.
Rai et al. (Rai et al. 2000) developed a model for Th(IV) complexation with ISA. The major feature of their model is the predominance of thorium ternary complexes, e.g., Th(OH)4ISA2 2- , not ThISA2 2+ complexes, as was proposed by Allard and Ekberg (Allard and Ekberg 2006a and Allard and Ekberg 2006b). According to Rai's model, mM ISA concentrations will not affect solubility of thorium. Data for higher ISA concentrations might be questionable, because Rai's model is based on 15 and 69 days equilibration times for ~10-6 M thorium concentrations. On the basis of our experiments and those published by German researchers, µM thorium concentrations can persist for years as a metastable state without ISA present. Complexes with a similar stoichiometry were also observed for uranium (VI) and the authors did not observe any enhanced solubility caused by ISA for pH in the range of 9.0 to 13.5 (Warwick et al. 2006).
For WIPP-related conditions, the occurrence of cellulosic chemical degradation pathways have a very low probability and, even if degradation occurs, the ISA formed will likely have a negligible effect on An(IV) solubility.
The effect of organic complexation on the An(III) and An(IV) oxidation state were evaluated under WIPP-relevant conditions. EDTA and citrate had a strong effect on the An(III) solubility, but had essentially no impact on An(IV).
The effect of organic complexation on the solubility of Th(IV), as the An(IV) actinide analog, was determined in GWB brine in the presence of inventory-predicted organic concentration. The simultaneous presence of four organic chelating agents (2.42 × 10-3 M acetate, 3.02 × 10-2 oxalate, 3.62 × 10-3 citrate and 9.28 × 10-5 EDTA) led to a measured thorium solubility of 7.34 × 10-7 M in GWB brine at pCH+ = 9.3. This is in agreement with the 2-year solubility data (Borkowski et al., 2012) of 5 × 10-7 M and there is an order of magnitude agreement with the recently calculated thorium solubility for CRA-2014 (Brush and Domski 2013a). These experimental data confirm that there is no significant effect on the measured thorium solubility due to the presence of the organic chelators at or near their inventory-predicted limits.
The effect of organic complexation on the concentration of neodymium, as the An(III) analog, was also evaluated for each key chelating agent. These data are shown in Figure SOTERM-21. These data show a strong effect of citrate and EDTA where a 1:1 complex with the neodymium is being formed and the concentration of the neodymium is approximately the concentration of EDTA in ERDA-6 brine and ~ 50% of the concentration of EDTA in GWB brine.
Figure SOTERM- 21. Effect of EDTA, Citrate, Oxalate and Acetate on the Solubility of Nd3+ in GWB Brine.
The potential for colloidal species to have a role in defining the solution concentration and mobility of actinides in the WIPP was recognized early in the WIPP licensing process. This led to the development of a colloid model that accounts for these colloidal species. This model was based on an extensive literature review, some WIPP-specific experimental data, and some conservative simplifications that were extensively peer reviewed prior to the first license application (CCA). In this model, four types of colloids that could contribute to the actinide source term are identified: intrinsic, mineral, microbial and humic. The EPA found this model and approach to be satisfactory in the WIPP certification and subsequently in the CRA-2004 and CRA-2009 recertification. There has been essentially no change in this model since its initial certification by the EPA.
Actinide colloids in the WIPP are potentially important since the actinide source term is defined by the WIPP PA as the sum of contributions from dissolved actinide species and mobile colloidal actinide species (see U.S. DOE 2004, Appendix SOTERM-2004, and Reed et al. 2013) for a more detailed discussion of WIPP-relevant colloids). The importance of colloids in the migration and transport of actinide contaminants, although it continues to receive attention in the literature, remains somewhat controversial and difficult to prove. In this context, the consideration of colloidal enhancement of actinide concentrations by the WIPP PA is, at least in part, a conservatism that is built into the overall PA approach. In this context, the sorption of colloidal actinides onto fixed substrates and their filtration in low-porosity media will also reduce the mobile colloidal actinide source term, but no credit is currently being taken for this potentially significant reduction in colloidal concentrations.
Actinide colloids or pseudocolloids may be generated in the WIPP repository as a result of
1. Hydrolysis (intrinsic chemistry).
2. The interactions of dissolved actinide species with microbially derived colloids or colloids formed due to the corrosion of steel and waste constituents.
3. The hydrodynamic entrainment of colloidal-sized mineral fragments, as well as several other mechanisms.
The formation of colloids could enhance actinide release in two ways. First, increased actinide concentration will increase the magnitude of DBR release and the effective actinide source term concentration for transport through the Culebra. Second, colloids have very different transport properties than dissolved species, and are predicted to migrate more rapidly in the subsurface. This transport mechanism could enhance the overall actinide release in the WIPP through migration pathways in the Culebra member and the Salado.
The current WIPP colloidal model defines four potential colloidal contributions to the mobile actinide concentration that comprises the actinide source term:
Four potential types of colloids are recognized in the CCA and these definitions have not changed since then.
1. Mineral fragments are hydrophobic, hard-sphere particles that are kinetically stabilized or destabilized by electrostatic forces, and may consist of crystalline or amorphous solids. Mineral fragments may be made kinetically stable by coatings with steric stabilizers that prevent close contact. Mineral fragments may act as substrates for sorption of actinides or they may consist of precipitated or coprecipitated actinide solids.
2. Actinide intrinsic colloids are macromolecules of actinides that, at least in some cases, may mature into a mineral-fragment-type of colloidal particle. When immature, they are hydrophilic; when mature, they become hydrophobic.
3. Humic substances are hydrophilic, soft-sphere particles that are stabilized by salvation forces. They are often powerful substrates for uptake of metal cations and are relatively small (less than 100,000 atomic mass units).
4. Microbes are relatively large colloidal particles that are stabilized by hydrophilic coatings on their surfaces, which behave as steric stabilizing compounds. They may act as substrates for extracellular actinide sorption or they may actively bioaccumulate actinides intracellularly.
In this section, the general environmental aspects of colloid-enhanced transport in the subsurface are discussed, along with an update of relevant WIPP-specific results since the CRA-2009 PABC.
The extent and potential formation of actinide colloids continues to be debated by researchers in the field. Since the CCA, there have been over 100 publications on actinide colloid chemistry that range in topics from real-system transport studies to the structure and inherent stability of actinide colloids. These remain largely focused on plutonium and its associated and very complex subsurface chemistry, but there are also studies on neptunium, americium, thorium and curium reported in the literature. It is also important to note that relatively few of these studies specifically address ionic-strength effects on colloid formation, stability and mobility. In this context, there are very few studies that in high ionic-strength systems (I > 5 M) and only a small fraction of these studies have direct application to the WIPP repository safety case.
A more extensive literature review is provided elsewhere (Reed et al. 2013). Key observations from the literature that impact the WIPP colloid model parameters are:
· A wide variety of actinide colloids are now noted to form in natural systems (see for example Khasanova et al. 2007). This differs somewhat from the conclusion made at the time of the CCA that only Pu colloids could form.
· Colloids that form in nature tend to be associated with iron colloidal species and tend to help immobilize rather than mobilize actinides. This is consistent with the WIPP model assumptions that only iron mineral colloids seem to form (see also new WIPP-specific date in section SOTERM-3.9.2). Colloidal species in the WIPP conceptualization primarily add to the source term concentration with only a small contribution to transport pathways through the Culebra.
· There are new data showing the existence of nanoclusters as an integral part of the aqueous speciation of some actinides. These are also seen in WIPP-specific brine systems (Reed et al. 2013; Section SOTERM-3.9.2).
· Bioassociation of actinides is observed in the literature and we have shown that this also extends to halophilic microorganisms (Figure SOTERM-22; Ams et al. 2013; Reed et al. 2013; Section SOTERM-3.9.2).
Figure SOTERM- 22. Experimental Data for Neptunium (V) Adsorption onto Chromohalobacter sp. as a Function of pH in 2 (Open Circles) and 4 (Open Triangles) M NaClO4. Adsorption experiments were performed with 5 x 10-6 M total neptunium (V) and 5 grams per liter (g/L) (wet weight) bacteria (Ams et al. 2013). Solid curves represent best-fit calculated surface complexation models. Solid diamonds, squares, triangles, and circles represent the results of desorption experiments performed with 5 x 10-6 M total neptunium (V) and 5 g/L (wet weight) bacteria in 2 M NaClO4.
Overall Impact of literature publications on the WIPP Colloid Model
Although there continues to be some progress made in the assessment of the colloidal issue as it applies to the potential subsurface migration of actinide species, there remains a great deal that is not well understood and substantive progress in this area is not likely in the very near future. The following general recommendations remain:
· It remains critical that the WIPP model continue to address the colloid issue.
· There is no literature evidence that the current four-colloid type model is inadequate; if anything it continues to be a conservative assumption built into the model.
· Current literature shows that colloidal species, intrinsic and mineral, of a number of actinides, not just plutonium, is observed - this is somewhat of a departure from the initial CCA literature survey conducted. These literature data, however, still do not explicitly address high ionic-strength systems.
· The structural data point towards intrinsic colloids that persist as very small (typically < 10nm) species.
· Biosorption data show that increased ionic-strength increased the extent of sorption and the overall trend with pH was to go through a maximum at about pH 8 and then decrease with increasing pH. At the predicted pCH+ of ~ 9.4 in the WIPP site, biosorption is in the range of ~ 40-65%.
The mineral, intrinsic and microbial contribution to the WIPP mobile colloidal actinide source term model was re-examined in light of recent literature results and new WIPP-specific data (Reed et al. 2013). An extensive amount of work under WIPP-specific conditions was completed to re-assess the microbial colloid enhancement parameters. There were some discrepancies in the model in this area due to the lack of extensive WIPP-specific data at the time of the CCA. These experiments build on the more extensive understanding that we now have about the microbial ecology in the WIPP. Microbial colloid enhancement parameters based on these new data are recommended and these are, in general, more realistic and lead to a lower overall contribution of microorganisms to the actinide source term.
As a whole, the WIPP-specific data obtained since CRA-2009 provide the first WIPP-specific data on colloids since the time of the CCA. These data, although not complete, provide significant improvement in our understanding of the potential contribution of colloidal species to the actinide source term. Additionally, some inconsistencies between the known solution chemistry and literature observation are addressed. Updated parameters (see discussion in Section SOTERM-4.0) for the intrinsic, mineral and microbial colloid enhancement parameters are recommended.
The intrinsic and mineral colloidal fraction of the actinides and analogs investigated as part of the long-term solubility studies was determined to provide WIPP-specific data. The size fractionation was determined using ultrafiltration and, in some cases, ultracentrifugation methods down to ~ 2.5 nm. In almost all cases, <10 nm size nanospecies were observed and assigned to the intrinsic colloidal fraction. The >10 nm size fraction was also established and used to evaluate the existence of mineral colloids.
The size distribution of aqueous species was shown for thorium and plutonium(III) in Figures SOTERM-10 and SOTERM-18 respectively. Additionally, results were obtained for neodymium (Figure SOTERM-23), uranium (Figure SOTERM-24) and plutonium in more detail (Figure SOTERM-25). In most cases, there was no filtration effect above 10 nm with the notable exception of plutonium where iron was also present in solution. There was no evidence for Mg-derived colloidal contributions.
Figure SOTERM- 23. Sequential Filtration Results for the Long-term Neodymium Solubility Studies in Brine (E = ERDA-6; G = GWB) as a Function of Filter Pore Size for Different pCH+ and Brines. Significant filtration effects are only noted for filters that are 10 nm or smaller in size.
Figure SOTERM- 24. Concentration of Uranium Measured during Sequential Filtration as a Function of Different Pore Size Filters for Different Brine Solutions at Different pCH+. Little/no filtration effect noted in all but one case above 10 nm filtration size.
Figure SOTERM- 25. Sequential Filtration Data for the Pu-Fe Experiments as a Function of Filtration at Different pCH+ and Brine Composition. GWB and ERDA-6 brine experiments contained excess iron powder with the exception of the "mag" designated experiment in ERDA-6 that contained excess magnetite.
The stability of humic and fulvic acid in the WIPP brine was investigated (Wall et al. 2005) and found to be unstable in the presence of MgO. These results add to the conservatism of the WIPP colloid model in that it shows that humic colloids are not likely in the WIPP. There is, however, no change in the model parameter assumptions for this colloidal species.
Experiments to measure the bioassociation of the An(III) and An(IV) actinides with WIPP-relevant microorganisms were performed. These experiments were focused on the biosorption of the two most important actinide oxidation states, Nd(III) for An (III) and Th(IV) for An(IV), towards a representative halophilic bacteria and archaea. Redox-invariant analogs were used so that there was no question about the oxidation state being sorbed. These biosorption data are used to recalculate the PROPMIC microbial colloid enhancement parameter and, when combined with laboratory observations of microbial growth under WIPP-relevant conditions, a biomass-based CAPMIC value (see Section SOTERM-5.4).
The microorganisms used in the biosorption experiments are indigenous to the WIPP area. The bacterium used, Chromohalobacter sp., was isolated from brine retrieved from a shallow subsurface monitoring well incubated under aerobic conditions (Swanson et al. 2012; Ams et al. 2013). Although these are incubated aerobically, from the point of view of biosorption, the DOE expects these to be representative of anaerobically derived species. The origins of this water are believed to be a mixture of seepage from an above-ground, but now capped, mine tailings salt pile and actual groundwater flow through the Santa Rosa and Dewey Lake contact (U.S. DOE 2011). Since then, this bacterium has also been isolated from incubations of WIPP halite at lower salt concentrations, and a Culebra groundwater incubated under aerobic, transitional, and nitrate-reducing conditions. The archaea utilized in these studies was Halobacterium noricense. This was isolated from incubations of halite in generic media and in the WIPP brines, detected in other subterranean salts worldwide (including Germany), requires 2.5-5 M NaCl, tolerates pH 6-10, and is 0.3-1.5 µm in size.
The biosorption experiments are described in detail elsewhere (Reed et al. 2013). The biosorption of actinides towards microorganisms should correlate with their aqueous speciation. For this reason, it would be expected that similar oxidation states would exhibit similar biosorption behavior, at least to the extent that their aqueous speciation is similar.
Example biosorption data are shown for thorium in Figure SOTERM-26. It is notable that the biosorption data are different for bacteria and archaea and EDTA complexation can reduce the extent of biosorption noted. In both +3 and +4 cases and in both Francis' and LANL-CO work, the PROPMIC values obtained for Archaea are less than those for Bacteria.
Changes in approach for An(III/IV) Biosorption Enhancement Parameters
Based on the current understanding of halophilism and microbial ecology at the WIPP site, microbial enhancement parameters were determined based on five observations made in the biosorption and microbial growth-related experiments.
· First, an emphasis on the archaeal data for the near field was used since this presented the most realistic scenario of which organisms will be present. In PA, however, bacterial values were used.
· Second, the biosorption data obtained at the lowest pH values investigated (~ pCH+ = 8.5) are most reliable and should be the basis of the PROPMIC calculation. These have well correlated biomass dependencies (so sorption is the predominant process) and overall concentration stability. The higher pH data for both thorium and neodymium appear to have significant contributions from precipitation pathways, although this is clearly more evident in the thorium data.
· Third, it is more consistent with the overall WIPP actinide model to use actinide oxidation state rather than element to assign biosorption enhancement parameters - although element-specific values are also provided. In the PA implementation, the element-specific values were used.
· Fourth, CAPMIC values were changed for all elements to a concentration based on microbial biomass and sorption capacity. This adds more realism to the model and accounts for variability in the toxicity data - which was used in prior CRAs to determine this value.
· Fifth, a biomass-based number was used for CAPMIC. The new biomass-based CAPMICs are less than the total mobile values in the case of the +3 oxidation state.
Figure SOTERM- 26. Biomass Dependency (top) and % Sorption (Bottom) of Thorium as a Function of pCH+ in pH-specific WIPP Brine. Reliance on lower-pH data was necessary due to the coupling of precipitation at the higher pHs investigated.
The calculation of the WIPP dissolved-actinide source term was performed for the CRA-2014 PA (Brush and Domski 2013a) using the computer code EQ3/6 (Wolery 1992; Wolery and Daveler 1992; Wolery 2008; Wolery, Xiong and Long 2010) version 8.0a and the database DATA0.FMT.R2 (Xiong 2011a). A general description of the modeling approach to establish the actinide source term for the WIPP PA is described in this section.
Changes since the CRA-2009 and CRA-2009 PABC
There are essentially no significant changes in the approach and overall conceptual model used to determine the solubility of actinides in the WIPP since CRA-2009. There are some changes in the mechanics of this process that are noted here:
· FMT is no longer used. All solubility calculations are done with EQ3/6. The qualification of EQ3/6 was done as a precursor to this transition (Wolery 2008; Wolery, Xiong and Long 2010; Xiong 2011b). The Pitzer data set of chemical equations has not changed.
· The colloid enhancement parameters for the actinides were updated based on new WIPP-specific data and published literature (see Section SOTERM-3.9).
· Variable brine volume is now being implemented in PA (see SOTERM-5.1.4 and Brush and Domski 2013a).
The overall approach used to establish the actinides important in WIPP releases and calculate their solubilities for use in the WIPP PA is summarized in this section. This approach consists of the following:
· Assess the WIPP inventory and regulations that govern the application of the WIPP certification to determine the likely actinides of interest and, correspondingly, the key waste components that may affect their solubility.
· Establish a conceptual model for the key subsurface interactions and release mechanisms and using a combination of literature review and WIPP-specific experimental results to establish the likely oxidation state distribution, the species that affect actinide solubility and the parameters required to model the system at high ionic strength. This approach featured the following:
- Conservative assumptions, within the bounds of the conditions expected, for the oxidation state distribution.
- Use of redox-invariant analogs for multivalent actinides to determine formation constants and establish oxidation-specific solubilities.
- Use of the Pitzer activity-coefficient model and associated parameters to model solubilities at the high ionic strengths present. The Pitzer approach is recognized as the best approach for I > 0.3 M in brine systems.
- Calculate the solubility of the key actinides in the WIPP using the EQ3/6 code. The solubilities are modeled in reacted GWB and ERDA-6 brines and include the effects of organic complexation. This is expected to bracket the range in the composition of the brine expected.
· Establish the effects of colloids on the solubilities calculated.
· Tabulate and assign uncertainty distributions in the range of expected conditions and brine compositions to these solubility data. A new method for this is used in CRA-2014 (see Brush and Domski 2013c).
This range of possible solubilities for a wide range of possible conditions defines the actinide source term provided to the WIPP PA for the calculation of TRU release from the WIPP.
The solubility and speciation of multivalent actinides are often investigated with lanthanide and actinide analogs that mimic the property of interest but, for varying reasons, provide an advantage to the experimenter. The best example of this, used extensively in the WIPP modeling approach, is the use of redox-invariant analogs for the multivalent actinides, most notably Pu, to determine oxidation-state-specific properties (e.g., solubility or complexation). The advantage of these types of analogs is that they remove the uncertainty of oxidation-state change from the experiment, which is a complexity that can often lead to uncertain or incorrect interpretations of the results obtained.
For the TRU actinides, the redox-invariant analogs used are lanthanides or other actinides. Lanthanides, as 4f-electron elements, possess physical and chemical characteristics that make them good analogs for the actinides when they are redox-invariant under the conditions of the experiment. Correspondingly, actinides with their 5f-electron character also have good physical and chemical properties to be analogs for other actinides if they also have redox stability under WIPP-relevant conditions. This analog approach, although sometimes criticized in the literature, considerably simplifies experimental design and consequently improves the reliability of the experimental data (Choppin 1999).
A key argument for the use of analogs in WIPP-related experiments is that key complexants that define actinide solubility in the WIPP are hard-donor complexants (e.g., hydroxide, carbonate, borate, chloride, and/or sulfate). The use of lanthanides as analogs for actinides is based on observations in many extraction systems, along with the associated crystallographic data (Siekierski 1988) that show they are good analogs for compounds containing hard donor ligands (oxygen) where the cation-anion interactions are primarily electrostatic in nature. In this context, Nd(III) is a good analog for the chemical behavior of Am(III) and Pu(III) under most circumstances in the WIPP. Not only do these species have the same 3+ charge, they also have similar ionic radii for coordination number 6 (CN=6): 97.5 pm for Am3+, 98.3 pm for Nd3+, and 100 pm for Pu3+ (Shannon 1976). In this context, the magnitudes of electrostatic attractions between these metal ions and corresponding ligands will be similar, yielding comparable thermodynamic stabilities.
Th is used by the WIPP as a redox-invariant analog for Pu(IV), U(IV), and Np(IV). The use of the Th4+ stability constants to represent the other An(IV) species is conservative. Th4+ is the largest of the tetravalent actinide ions. It therefore has the lowest charge density and, correspondingly, relatively weaker ionic interactions when compared to the other tetravalent actinides. This is best exhibited by its lower tendency towards hydrolysis and intrinsic polymer formation relative to the other actinides (see Section SOTERM-3.2). For these reasons, the use of Th4+ as an analog is conservative, as Th will likely be the most soluble of the actinides in the tetravalent state under comparable WIPP-relevant conditions.
To a lesser extent, actinides are analogs for each other, depending on the oxidation state. Np(V), which has much greater redox stability than Pu(V) and much more favorable spectroscopy, is often used as an analog for Pu(V). U(VI), which is much more redox stable than Pu(VI) and Np(VI), is also used as an analog for these TRU actinides, although U(VI) is in fact a poor analog for Pu(VI) solubility. Am(III) and Cm(III) are also excellent analogs for Pu(III) as a result of their much greater redox stability and comparable ionic radii.
The actinide inventory used in CRA-2014 PA was the 2012 inventory that was summarized in Van Soest (Van Soest 2012). Key aspects of this were provided in Tables SOTERM-10 and SOTERM-11.
The oxidation states used by the WIPP PA to model actinide solubility are tabulated in Table SOTERM-17. Also included are the assumed abundance percent of each oxidation state and the speciation data set used in EQ3/6 for each oxidation state. This table is based on a general understanding of the corresponding actinide chemistry summarized in Section SOTERM-3.0.
Table SOTERM- 17. Oxidation States of the Actinides in the WIPP as Used in the CRA-2014 PA
|
Actinide Element |
Oxidation States, Abundance (%), and Analog Used (If Any) |
||||
|
Oxidation Statea,b |
EQ3/6 Speciation Data Used |
||||
|
III |
IV |
V |
VI |
||
|
Thorium |
- |
100 % |
- |
- |
Thorium |
|
Uranium |
- |
50 % |
- |
50 % |
1 mM assumed for VI, |
|
Neptunium |
- |
50% |
50 % |
- |
Np for V |
|
Plutonium |
50 % |
50 % |
- |
- |
Am for III |
|
Americium |
100 % |
- |
- |
- |
Americium |
|
Curium |
100 % |
- |
- |
- |
Americium |
|
a Oxidation state distributions (percentages) refer to the percent of PA vectors that have 100% of the specified oxidation state. b In PA calculations the distribution of oxidation states is correlated for U, Np, and Pu such that the states for all three elements are simultaneously either in the lower oxidation state (U(IV), Np(IV), and Pu(III)) or in the higher oxidation state (U(VI), Np(V), and Pu(IV)). |
|||||
A number of conservative assumptions are reflected in this table:
1. Use of 1 mM concentration for the solubility of U(VI). The actual solubility of U(VI) in the WIPP under the expected range of conditions is estimated to be <0.1 mM.
2. Use of Th as an analog for the IV actinides (see Section SOTERM-4.1 and Section SOTERM-3.2).
3. The assumption of a probability that 50% of the vectors have Pu(III) and 50% of the vectors have Pu(IV). The predominant Pu species expected is Pu(IV), although some Pu(III) is possible as a transient (see discussion in Section SOTERM-3.6). This is conservative because Pu(III) is approximately 6 to 10 times more soluble than corresponding Pu(IV) phases.
4. The assumption of a probability that 50% of the vectors have U(IV) and 50% of the vectors have U(VI). The predominant uranium species expected is U(IV), which is approximately four orders of magnitude less soluble than U(VI), based on current assumptions.
The version of the database used with the EQ3/6 code for the CRA-2014 PA was DATA0.FMT.R2 (Xiong 2011a). This was a conversion of the FMT database used in CRA-2009 into the EQ3/6 format and was extensively qualified (Wolery, Xiong and Long 2010). There were no significant changes to the speciation reactions and data used. For these reactions (see Wolery 1992 for a detailed discussion), log K is the log of the product of the activity of each reaction product (to the power of its coefficient) divided by the product of the activity of each reactant (to the power of its coefficient).
The thermodynamic database for the III actinides currently used in EQ3/6 was described by Giambalvo (Giambalvo 2002a) and updated by Wolery (Wolery, Xiong and Long 2010). Nd, Am, and Cm are generally used to establish solubility of An(III) because, unlike plutonium, they have redox-stable trivalent oxidation states. Speciation and solubility data for the III actinides were parameterized for use in the Pitzer activity-coefficient model by Felmy et al. (Felmy et al. 1989) for the Na+- Pu3+-Cl--H2O system; by Felmy, Rai, and Fulton (Felmy, Rai, and Fulton 1990) for the Na+-Am3+-OH--HCO3 --H2O system; by Rai, Felmy, and Fulton (Rai, Felmy, and Fulton 1995) for the Na+-Am3+-PO4 3--SO4 2--H2O system; and by Rao et al. (Rao et al. 1996) for the Na+-Nd3+-CO3 2--HCO3 --H2O system. EQ3/6 uses the Am(III) data to calculate the solubility for all the III actinides. A diagram of the predominant species for Am is shown in Figure SOTERM-27.
The inorganic aqueous and solubility-limiting species featured in the model for Am(III) are
|
Am(III) Reactions |
log K |
|
|
In these reactions, "aq," "cr," and "s" are the abbreviations for aqueous, crystalline, and solid, respectively. The An(III) database was extended to mixed Na+-CO3 2--Cl-- media, and was shown to reproduce the independently measured solubility of NaAm(CO3)2(s) in 5.6 M NaCl (Runde and Kim 1994) and the measured Nd(III) solubility in the WIPP brine (Borkowski et al. 2008).
Figure SOTERM- 27. Predominant Am Species as a Function of pH and Eh Based on the Speciation Reactions 34 to 47 (Richmann 2008)
The IV actinides addressed by the WIPP PA are Th(IV), U(IV), Pu(IV), and Np(IV). The variation in charge-to-radius ratio for the tetravalent actinides is greater than for actinides in other oxidation states (Cotton and Wilkinson 1988, pp. 11-46), and larger differences in the chemical behavior among the IV actinides is expected. The application of the Th(IV) model to the other IV species (U(IV), Np(IV), and Pu(IV)) is more uncertain, yet still conservative because Th(IV) is the most soluble of these elements under WIPP conditions. The model was evaluated against data for Pu(IV) and Np(IV) solubility and demonstrated to predict the chemical behavior of these actinides conservatively.
The thermodynamic database for the IV actinides currently used in EQ3/6 was described by Giambalvo (Giambalvo 2002b). Speciation and solubility data for Th(IV) were parameterized for the Pitzer activity-coefficient model for the Na+-K+ -Mg2+-Cl-- SO4 2--CO3 2--HCO3 - -OH--H2O system. This model requires the species Th4+, Th(OH)2SO4 (s), Th(SO4)3 2-, Th(SO4)2 (aq), ThO2, Th(OH)4(aq), Th(OH)3CO3 -, and Th(CO3)5 6- to describe the data pertinent to the WIPP (Felmy, Mason, and Rai 1991; Rabindra et al. 1992; Felmy et al. 1996). A diagram of the predominant Th speciation, based on Reactions SOTERM.48 to 59, is shown in Figure SOTERM-28.
Figure SOTERM- 28. Predominant Species of Th as a Function of pH and Redox Conditions (Richmann 2008). Thorianite is predicted to predominate at the conditions expected in the WIPP repository.
The inorganic aqueous and solubility-limiting species featured in the IV model are:
|
Th(IV) Reactions |
log K |
|
The only V actinide of interest to the WIPP is Np(V), which exists as the neptunyl ion, NpO2 +. Pu(V), which can be formed under some conditions, is transitory and not expected to persist in significant quantities in the WIPP. The base model for Np(V) comes from Fanghänel, Neck, and Kim (Fanghänel, Neck, and Kim 1995), constructed for the German repository program.
The thermodynamic database for the V actinides currently used in EQ3/6 is described by Giambalvo (Giambalvo 2002c). Np(V) speciation and solubility were parameterized in the Pitzer activity-coefficient model for the Na+-K+ -Mg2+-Cl-- SO4 2--CO3 2--HCO3 - -OH--H2O system. The model requires the aqueous species NpO2 +, NpO2OH(aq), NpO2(OH)2 -, NpO2CO3 -, NpO2(CO3)2 3-, and NpO2(CO3)3 5-, and the solid species NpO2OH(am), NpO2OH(aged), Na3NpO2(CO3)2(s), KNpO2CO3 ×2H2O(s), K3NpO2(CO3)2 ×0.5H2O(s), and NaNpO2CO3 ×3.5H2O(s) to explain the available data. The predominant species for Np(V) are shown in Figure SOTERM-29.
The inorganic aqueous and solubility-limiting species used are:
|
Np(V) Reactions |
log K |
|
|
|
(SOTERM.60) |
|||
|
|
|||
|
|
|||
|
|
|||
|
|
|||
Figure SOTERM- 29. Predominant Species Diagram for Np as a Function of pH and Eh Based on the Np Speciation Data Reactions 60 to 70 (Richmann 2008)
The An(VI) EQ3/6 model has not been developed sufficiently for reliable use in predicting concentrations of this oxidation state in the WIPP brines under various solution conditions. Although uranyl carbonate can be successfully modeled, the hydrolysis behavior of U(VI) is quite complicated and no satisfactory predictive models applicable to WIPP-like conditions are yet available. Because the implementation of an MgO backfill limits the pmH and fCO2 to discrete values, empirical measurement of the solubility of U(VI) in WIPP and/or WIPP-like brines became practical. As documented in Hobart and Moore (Hobart and Moore 1996) and used in prior PA calculations, the solubility of U(VI) at pH 10, in the absence of carbonate, was determined to be 8.8 ´ 10-6 m. This is augmented by additional data from U(VI) solubility studies in WIPP-relevant carbonate-free brines reported in Section SOTERM-3.3.2 (Lucchini et al. 2010a; Lucchini et al. 2013a; Lucchini et al. 2013b). Here, the measured U(VI) solubility was 10-7 M to 10-6 M for GWB and ERDA-6 brine, respectively. The solubility of U(VI) currently used in the WIPP PA was established through discussions with the EPA to be 1 mM (U.S. EPA 2005) to account for the potential and expected effects of carbonate.
Details of the implementation of EQ3/6 are described in more detail elsewhere (Wolery 2008; Wolery and Jarek 2003; Wolery, Xiong and Long 2010; Brush and Domski 2013a). EQ3/6 calculates chemical equilibrium for user-specified total element amounts in aqueous or aqueous/mineral geochemical systems. The EQ3/6 calculations of actinide solubility in the WIPP system performed for the WIPP PA included preequilibration with halite, anhydrite, brucite, and hydromagnesite (Brush and Domski 2013a), which are the minerals present in large quantities in the repository. The effects of the MgO backfill are realized by equilibrating brine with brucite, magnesite, and hydromagnesite.
The Pitzer activity-coefficient model is substantially different in approach from the classic Debye-Hückel (D-H) theory of the behavior of ionic solutions. The latter is a theoretical approach to describing the behavior of dilute solutions; more importantly, because many ionic solutes do not behave ideally even at very low concentrations, D-H provides a means to calculate the activity, ai , of a desired species. This is of great importance, as the Gibbs free energies of the various species in solution can be used to calculate solution equilibria if one knows the effective concentration of those species, i.e., their "activity" in solution. The activity of a given species i is tied to the molality of that species as ai = γimi . Since the molality of species i is known, the unknown that must be calculated to determine ai is, therefore, γi . The simplest form relating activity to molality from the D-H law is
where Aγ is the Debye-Hückel parameter, zi is the charge of the ith species and I is the overall solution ionic strength. The fundamental difficulty with the D-H formalism is that even with extensions (Davies equation, B-dot equation)(Wolery 2008), the D-H law begins to deviate significantly from real solution behavior somewhere in the general region of I = 0.3 molal. As the WIPP brines (and many other highly concentrated ionic species of interest) are well above this level of ionic strength, many times with I > 5, another description is required to properly describe the activities of the ionic species.
In 1973, Pitzer proposed a set of semiempirical equations to describe ai . Pitzer (Pitzer 1973) wrote the Gibbs excess energy of a solution as a virial expansion, where a portion of the overall expansion can be tied down to a formalism similar to the D-H law and the majority of the remaining constants are empirically determined from measurements of the desired ions. The most general form of the equation is
where f(I) is a Debye-Hückel function, f ¢(I) is its derivative df/dI, the λij are second-order interaction coefficients, λ'ij(I) is the derivative dλij/dI, and the μijk are third-order interaction coefficients. The experimentally observable values β(0), β(1), β(2), α1, α2, Cφ, and so forth are used to calculate the λij and μijk values needed to calculate γi (for more detail, see Wolery and Daveler 1992).
This approach has proven highly effective and has successfully described the behavior of solutions at high ionic strength. The disadvantage of this technique is that binary and ternary coefficients for the expansion are normally needed to completely describe all the activities of the different species; in addition, if the number of species in solution grows, the number of calculations grows that much faster, i.e., on the order of the cube of the number of species. This problem would be even worse, except that many of the terms describing neutral species can be legitimately neglected in geochemical systems.
This parameter-determination problem is of particular interest in the description of actinide behavior in the WIPP, since the GWB and ERDA-6 brines of interest contain a wide variety of ions in and of themselves, in addition to the actinides introduced into the repository. As a result of this, it was necessary to constrain the total number of possible species in solution, aqueous, solid or gas, and in addition, to determine Pitzer parameters for many species by analogy to others rather than by experimental measurement. This is the basis of the parameter and species selection in the current database, DATA0.FMT.R2, which contains the parameters for those species incorporated into the limited species set description. In practice, this has worked well to describe solution behavior in the WIPP within a limited set of pH values at 25 oC.
The oxidation-state-specific actinide solubilities calculated for the CRA-2014 PA with EQ3/6 are summarized in Table SOTERM-18. For historical perspective, the calculated solubilities from prior PA analyses are also tabulated. In the CRA-2014 PA, the data are shown for two brines in the presence of organics, and as a function of equilibration with hydromagnesite. The hydromagnesite case is recognized by the project as the most relevant to the WIPP. It is important to note that, overall, the calculated solubilities have not changed much over time except for the effects of increased complexation of the An(III) actinides with organics as this inventory has increased.
As shown in Table SOTERM-18, the calculated solubility of the III actinides was 2.59 ´ 10-6 M to 1.48 ´ 10-6 M in the CRA-2014 PA (Brush and Domski 2013a). These data are also fairly consistent with recently measured results for Nd(III) solubility in brine (Borkowski et al. 2008). A somewhat broader range was noted historically: 2.88 ´ 10-7 M to 2.59 ´ 10-6 M. The expected solubility of the IV actinides ranges between 6.05 ´ 10-8 M and 7.02 ´ 10-8 M. This is also somewhat consistent with prior calculations and has increased slightly. Overall the solubility of the IV actinides is four to eight times lower than that predicted for the III actinides. The main reason for increases noted in CRA-2014 PA was the presence of organics in the brines.
Uncertainties in the solubility data and uncertainty in the NONLIN least-squares refinement, for Pitzer parameter determination, result in uncertainty in the model predictions. This distribution was sampled and used in PA as discussed in Section SOTERM-5.0 (Brush and Domski 2013c).
Four organic ligands are included in EQ3/6 calculations of actinide solubilities. These are acetate (CH3CO2 -), citrate [(CH2CO2)2C(OH)(CO2)3-], EDTA [(CH2CO2)2N(CH2)2N(CH2CO2)2 4-], and oxalate (C2O4 2-). The current anticipated inventory of these complexing agents, with their inventory-limited solubilities in the WIPP, were summarized in Tables SOTERM-3 and SOTERM-7. These ligands are included in the solubility calculations because (1) approximately 60 organic compounds were identified among the nonradioactive constituents of the TRU waste to be emplaced in the WIPP (Brush 1990; Drez 1991; U.S. DOE 1996); (2) 10 of these 60 organic compounds could, if present in the WIPP, increase actinide solubilities because they are soluble in aqueous solutions such as the WIPP brines, and because they form complexes with dissolved actinides (Choppin 1988); and (3) of these 10 water-soluble organic ligands that form complexes with actinides, 4 (acetate, citrate, EDTA, and oxalate) are included in PA and tracked in the WIPP inventory (see the CCA, Appendix SOTERM, p. 96).
Table SOTERM- 18. Historical Actinide Solubilities Calculated for the CRA-2004 PABC, the CRA-2009 PABC and CRA-2014 PA (Brush and Domski 2013a, Table 13).
|
Actinide Oxidation State, and Brine |
CRA-2004 PABC (M) |
CRA-2009 PABC (M) |
CRA-2014PA, 1× Minimum Brine Volume (M) |
CRA-2014 PA, 5× Minimum Brine Volume (M) |
|
III, GWB |
3.87 ´ 10-7 |
1.66 ´ 10-6 |
2.59 ´ 10-6 |
6.47 ´ 10-7 |
|
III, ERDA-6 |
2.88 ´ 10-7 |
1.51 ´ 10-6 |
1.48 ´ 10-6 |
3.92 ´ 10-7 |
|
IV, GWB |
5.64 ´ 10-8 |
5.63 ´ 10-8 |
6.05 ´ 10-8 |
6.07 ´ 10-8 |
|
IV, ERDA-6 |
6.79 ´ 10-8 |
6.98 ´ 10-8 |
7.02 ´ 10-8 |
7.20 ´ 10-8 |
|
V, GWB |
3.55 ´ 10-7 |
3.90 ´ 10-7 |
2.77 ´ 10-7 |
1.82 ´ 10-7 |
|
V, ERDA-6 |
8.24 ´ 10-7 |
8.75 ´ 10-7 |
8.76 ´ 10-7 |
6.44 ´ 10-7 |
The importance and role of colloids in defining the concentration of actinides in the WIPP was discussed in Section SOTERM-3.9, and more extensive discussions of WIPP-related results are available (Reed et al. 2013; CCA Appendix SOTERM, Section 6 ). The PA conceptual approach used to account for colloidal enhancement of actinide concentrations was developed as part of the CCA and has not changed since this initial implementation. The four types of colloids identified as relevant to the WIPP are listed and described in Table SOTERM-19.
Three types of parameter values were determined: (1) constant concentration values, (2) concentration values proportional to the dissolved actinide concentration, and (3) maximum concentration values. These parameter types are summarized Table SOTERM-20 and were initially described in parameter record packages (Papenguth and Behl 1996; Papenguth 1996a; Papenguth 1996b; Papenguth 1996c).
For microbes, the proportionality relationship was made by element. For humic actinides, however, the relationship was made by oxidation state, rather than by element. For microbes and humic substances, the experiments described in the parameter record packages noted above also provided a basis to define upper limits of the actinide concentration that could be associated with each of those colloid types. For both humic and microbial actinides, the upper limit parameter was defined by element, rather than oxidation state, and is in units of molality. The use of the two upper limit parameters is slightly different, and is described in the sections below discussing humic substances and microbes.
Table SOTERM- 19. Classification of Four Colloid Types Considered by the WIPP PA
|
Mineral Fragment Colloids |
Hydrophobic, hard-sphere particles that are kinetically stabilized or destabilized by electrostatic forces and may consist of crystalline or amorphous solids. Mineral fragments may be made kinetically stable by coatings with steric stabilizers that prevent close contact. Mineral fragments may act as substrates for sorption of actinides, or they may consist of precipitated or coprecipitated actinide solids. |
|
Intrinsic Actinide Colloids |
Intrinsic actinide colloids (also known as true colloids, real colloids, Type I colloids, and Eigenkolloide) are macromolecules of actinides that, at least in some cases, may mature into a mineral-fragment type of colloidal particle. When immature, they are hydrophilic; when mature, they become hydrophobic. |
|
Humic Colloids |
Humic substances are hydrophilic, soft-sphere particles that are stabilized by solvation forces. They are often powerful substrates for uptake of metal cations and are relatively small (less than 100,000 atomic mass units). |
|
Microbial Colloids |
Microbes are relatively large colloidal particles stabilized by hydrophilic coatings on their surfaces, which behave as steric stabilizing compounds. They may act as substrates for extracellular actinide sorption or actively bioaccumulate actinides intracellularly. |
Table SOTERM- 20. Material and Property Names for Colloidal Parameters
|
Material |
Property |
Brief Description of Parameter |
|
Th, U, Np, Pu, Am |
CONCMIN |
C oncentration of actinide associated with mobile mineral fragment colloids |
|
Th, U, Np, Pu, Am |
CONCINT |
C oncentration of actinide associated with mobile intrinsic actinide colloids |
|
Th, U, Np, Pu, Am |
PROPMIC |
P roportionality constant for concentration of actinides associated with mobile microbes |
|
PHUMOX3
a
|
PHUMCIM |
P roportionality constant for concentration of actinides associated with mobile humic colloids; in Castile brine; actinide solubilities include organics (complexes with man-made organic ligands); solubilities were calculated assuming equilibrium with Mg-bearing minerals (brucite and hydromagnesite) |
|
PHUMOX3
a
PHUMOX6 |
PHUMSIM |
P roportionality constant for concentration of actinides associated with mobile humic colloids; in Salado brine; actinide solubilities include organics (complexes with man-made organic ligands); solubilities were calculated assuming equilibrium with Mg-bearing minerals (brucite and hydromagnesite) |
|
Th, U, Np, Pu, Am |
CAPMIC |
M aximum (cap) concentration of actinide associated with mobile microbes |
|
Th, U, Np, Pu, Am |
CAPHUM |
M aximum (cap) concentration of actinide associated with mobile humic colloids |
|
a Proportionality constant for actinide concentrations associated with mobile humic substances for PHUMOX3, for actinide elements with oxidation state III (that is, Pu(III) and Am(III)); PHUMOX4, oxidation state IV (Th(IV), U(IV), Np(IV), and Pu(IV)); PHUMOX5, oxidation state V (Np(V)); and PHUMOX6, oxidation state VI (U(VI)). |
||
The colloid concentration factors used in the CRA-2014 PA are summarized in Table SOTERM-21. The general approach used to account for colloidal enhancement of actinide solubilities is described in detail in Appendix SOTERM-2014, Section 5.2 and Appendix PA-2014, Section 4.3. There were essentially no changes in the approach used from the CRA-2009 PABC although all the parameters were re-assessed.
Table SOTERM- 21. Colloid enhancement parameters used in CRA-2009 and CRA-2014 (Appendix SOTERM-2009; Reed et al. 2013)
|
Actinide |
CONCMIN |
CONCINT (M) |
PROPMIC |
CAPMIC (M) |
Proportion Sorbed on Humicsb |
CAPHUMd
Humicsa) |
||||
|
PHUMSIM |
PHUMCIM |
|||||||||
|
CRA |
2009 and 2014 |
2009 |
2014 |
2009 |
2014 |
2009 |
2014 |
2009 and 2014 |
2009 and 2014 |
2009 and 2014 |
|
Th(IV) |
2.6 ´ 10-8 |
0 |
2 ´ 10-8 |
3.1 |
1.76 |
0.0019 |
2.3 x 10-6 |
6.3 |
6.3 |
1.1 ´ 10-5 |
|
U(IV) |
2.6 ´ 10-8 |
0 |
2 ´ 10-8 |
0.0021 |
1.76 |
0.0021 |
2.3 x 10-6 |
6.3 |
6.3 |
1.1 ´ 10-5 |
|
U(VI) |
2.6 ´ 10-8 |
0 |
3 ´ 10-8 |
0.0021 |
1.76 |
0.0021 |
2.3 x 10-6 |
0.12 |
0.51 |
1.1 ´ 10-5 |
|
Np(IV) |
2.6 ´ 10-8 |
0 |
2 ´ 10-8 |
12.0 |
1.76 |
0.0027 |
2.3 x 10-6 |
6.3 |
6.3 |
1.1 ´ 10-5 |
|
Np(V) |
2.6 ´ 10-8 |
0 |
ND |
12.0 |
1.76 |
0.0027 |
2.3 x 10-6 |
9.1 ´ 10-4 |
7.4 ´ 10-3 |
1.1 ´ 10-5 |
|
Pu(III) |
2.6 ´ 10-8 |
1 ´ 10-9 |
2 ´ 10-8
|
0.3 |
1.76 |
6.8 ´ 10-5 |
2.3 x 10-6 |
0.19 |
1.37e |
1.1 ´ 10-5 |
|
Pu(IV) |
2.6 ´ 10-8 |
1 ´ 10-9 |
2 ´ 10-8
|
0.3 |
1.76 |
6.8 ´ 10-5 |
2.3 x 10-6 |
6.3 |
6.3 |
1.1 ´ 10-5 |
|
Am(III) |
2.6 ´ 10-8 |
0 |
4 ´ 10-9 |
3.6 |
0.32 |
1.0 |
3.1 x 10-8 |
0.19 |
1.37e |
1.1 ´ 10-5 |
|
a In units of moles colloidal actinide per liter - 2009 and 2014 parameters are the same b In units of moles colloidal actinide per mole dissolved actinide c In units of moles total mobile actinide per liter d Humic colloid parameters for CRA-2009 and CRA-2014 are unchanged e At 0.5 probability NOTE: The colloidal source term is added to the dissolved source term to arrive at a total source term. Mineral fragments were provided with distributions, but the maximum was used as described in Appendix PA-2014, Section 8.4 |
||||||||||
The WIPP ASTP provided the parameters to construct the maximum dissolved and suspended colloidal actinide concentrations for use in modeling the mobilization and transport of actinides in the disposal system. In the WIPP PA, mobilization of radionuclides is represented by the PANEL code and transport of radionuclides within the repository and the Salado is represented by the Nuclide Transport System (NUTS) code (Appendix PA-2014, Section 6.7.3 and Section PA-6.7.2, respectively). A description of the simplifications, manipulations, and approach used in the PA to perform this modeling is discussed in this section.
The DOE has concentrated on those processes most likely to have a significant impact on system performance. Therefore, several simplifications were used in the modeling of radionuclide mobilization and transport in the CCA PA, the CCA PAVT, the CRA-2004 PA, the CRA-2004 PABC, the CRA-2009 PA, the CRA-2009 PABC and the CRA-2014 PA calculations. These include
· Using constant solubility parameters and constant colloidal parameters throughout the repository and regulatory period for a given realization
· Modeling only the isotopes most important to compliance
· Using the compositions of Castile and Salado brines (the end-member brines) to bracket the behavior of mixtures of these brines within the repository
· Sampling only the uncertain parameters with the most significant effect on repository performance
· Combining dissolved and colloidal species for transport within the disposal system, as modeled by NUTS and PANEL
Selection of isotopes for modeling mobilization and transport in the disposal system with NUTS and PANEL is described in Appendix PA-2014, Section PA-8.4. Runs of PANEL, the PA code that computes total mobilized radionuclide concentrations, include 29 radionuclides in the decay calculations (Kim 2013b, Table 3 and Table 11). Runs of NUTS, the PA code that computes radionuclide transport within the Salado, are based on five radionuclides (230Th, 234U, 238Pu, 239Pu, and 241Am) that represent groupings of radionuclides with similar decay and transport properties (Kim 2013a; Appendix PA-2014, Section PA-4.3.2 ). The number of radionuclides for transport calculations in NUTS has been reduced because calculations for the full WIPP inventory and decay chains would be very time consuming and because accurate results can be achieved with this limited set of radionuclides (Kicker and Zeitler 2013, Section 4 ).
Transport calculations in the Culebra use a reduced set of four radionuclides (230Th, 234U, 239Pu, and 241Am) for computational efficiency (Garner 1996). 238Pu has been omitted from transport in the Culebra because its short half-life (87.7 years) means that little 238Pu will enter the Culebra via brine flows up a borehole.
The general scenarios described in Appendix PA-2014, Section PA-2.3.2.2 and Section PA-8.3 and considered in the source term calculations may be categorized into three groups: (1) undisturbed performance (BRAGFLO S1 scenario); (2) intrusion through the repository and into the Castile, intersecting a pressurized brine reservoir (BRAGFLO S2, S3, and S6 scenarios); and (3) intrusion through the repository, but not into a pressurized brine reservoir (BRAGFLO S4 and S5 scenarios). The specific scenarios and the associated type of borehole intrusion considered by the WIPP PA are listed in Table SOTERM-22.
Table SOTERM- 22. WIPP PA Modeling Scenarios for the CRA-2014 PA (Garner and Leigh 2005; Leigh et al. 2005; Kim 2013a)
|
BRAGFLO |
Description |
Brine Used in PA |
|
S1 |
E0 (Undisturbed Repository) |
Salado (GWB) |
|
S2 |
E1 intrusion at 350 years penetrates the repository and a brine pocket |
Castile (ERDA-6) |
|
S3 |
E1 intrusion at 1000 years penetrates the repository and a brine pocket |
Castile (ERDA-6) |
|
S4 |
E2 intrusion at 350 years penetrates the repository (only) |
Salado (GWB) |
|
S5 |
E2 intrusion at 1000 years penetrates the repository (only) |
Salado (GWB) |
|
S6 |
E2 intrusion at 1000 years penetrates the repository (only); E1 intrusion at 2000 years penetrates the repository and a brine pocket |
Castile (ERDA-6) |
Brine may enter the repository from three sources, depending on the nature of the borehole intrusion. Under all scenarios, brine may flow from the surrounding Salado through the DRZ and into the repository in response to the difference between the hydraulic head in the repository and in the surrounding formation. For the BRAGFLO S2 through S6 scenarios, in which a borehole is drilled into the repository, brine may flow down the borehole from the Rustler and/or the Dewey Lake. For the BRAGFLO S2, S3, and S6 scenarios, in which a pressurized Castile brine reservoir is intercepted, brine from the Castile may flow up the borehole into the repository.
As mentioned in Section SOTERM-2.3.1, the brines in the Salado and Castile have different compositions and the actinide solubilities are somewhat different in each of these end-member compositions.
The composition of the more dilute groundwaters from the Rustler and Dewey Lake are expected to change rapidly upon entering the repository as a result of fast dissolution of host Salado minerals from the walls and floor of the repository. These minerals comprise about 90-95% halite and about 1-2% each of polyhalite, gypsum, anhydrite, and magnesite (Brush 1990). Calculations titrating Salado rock into dilute brines using EQ3/6 (Wolery 1992; Wolery and Daveler 1992) show that gypsum, anhydrite, and magnesite saturate before halite. When halite saturates, the brine composition is very similar to that of Castile brine. One hundred times as much polyhalite must be added to the system before the resulting brine has a composition similar to Salado brines. These calculations indicate that if dilute brines dissolve away only the surfaces of the repository, they will obtain Castile-like compositions, but if they circulate through the Salado after saturating with halite, they may obtain compositions similar to Salado brine. Similarly, if Castile brine circulates through enough host rock, it may also approach Salado brine composition. In either case, the actual brine within the repository may be described as a mixture of the two concentrated-brine end members: Salado and Castile. This mixture, however, is very hard to quantify, because it is both temporally and spatially variable. Only in the undisturbed scenario is the mixture well defined as 100% Salado brine over the 10,000-year regulatory period. In this context, the Salado (GWB) and Castile (ERDA-6) brines bracket the range of expected brine compositions.
For a panel intersected by a borehole, the BRAGFLO calculations show that in the 10% of the repository represented by the BRAGFLO panel computational cells, the ratio of brine inflow that enters through the borehole versus through inflow from the host rock varies in time and depends on the sampled parameter values and scenario considered. This ratio was the only measure of brine mixing available to the source term runs in the CCA PA, the CCA PAVT, the CRA-2004 PA, the CRA-2004 PABC, the CRA-2009 PA, the CRA-2009 PABC, and the CRA-2014 PA calculations. As an estimate, this ratio (1) does not account for compositional changes that occur when H2O is consumed by corrosion reactions or MgO hydration reactions; (2) does not resolve the details of flow, diffusion, and brine interaction with internal pillars and the DRZ; and (3) is an average over one-tenth of the repository. It is expected that the fraction of Salado brine will be quite high in areas of the repository distant from the borehole and much lower near the borehole. Because radionuclide travel up the borehole can lead to significant release, the solubility of radionuclides near the borehole is important. Given these uncertainties, the DOE decided to use the Castile end-member composition to calculate radionuclide solubilities for scenarios where a borehole penetrates a brine reservoir, and to use the Salado end-member composition for scenarios where it does not (see Table SOTERM-22).
The uncertain parameters to be sampled for the PA were selected based on the expected significance of their effect on repository performance. The following four parameters are sampled independently (Kim 2013a):
· The solubility uncertainty for oxidation state III (see discussion below and Figure SOTERM-30).
· The solubility uncertainty for oxidation state IV (see discussion below and Figure SOTERM- 31).
· The oxidation state for Pu, Np, and U. The sampled value is a flag that is "low" 50% of the time and "high" 50% of the time. If the flag is set to "high," Pu is assumed to be in the IV oxidation state, Np is assumed to be in the V oxidation state, and U is assumed to be in the VI oxidation state. If the flag is set to "low," Pu is assumed to be in the III oxidation state and Np and U are assumed to be in the IV oxidation state.
· The humic acid proportionality constant for the III oxidation state in Castile brine (see Table SOTERM-21 and Figure SOTERM-32).
Figure SOTERM- 30. Frequency Distribution of the Difference of Experimental log Solubility (log10Sm) from Model-Predicted Value (log10Sp) for Nd(III) and Am(III). A total of 243 measured and predicted solubilities were compared (Brush and Domski 2013c).
As discussed by Garner and Leigh (Garner and Leigh 2005, Section 2.3 ), the solubility uncertainty for oxidation state V is zero. There is no uncertainty assigned to the solubility for oxidation state VI because the EPA specified a fixed, maximum solubility of 1 × 10-3 mol/L for U(VI).
Actinide solubilities for a single realization in the PA depend on (1) the oxidation state; (2) the brine for that realization (see Table SOTERM-22); and (3) the solution concentration uncertainty, as shown in Equation (SOTERM.73).
Figure SOTERM- 31. Frequency Distribution of the Deviation of Experimental log Solubility from Model-Predicted Value for all An(IV) Comparisons. A total of 45 measured and predicted solubilities were compared (Brush and Domski 2013c).
Ci,b = (Si,b) ´ (10SUi ) (SOTERM.73)
Ci,b , used for every element in oxidation state i, is the concentration of oxidation state i and brine b. S i,b is the solubility calculated for oxidation state i in brine b with EQ3/6 (see Table SOTERM-18). SUi is the solubility uncertainty sampled from a distribution unique to each oxidation state. Figure SOTERM-30 shows the distribution of SU values for oxidation state III. Figure SOTERM-31 shows the distribution of SU values for oxidation state IV. These distributions are calculated and documented in Brush and Domski (Brush and Domski 2013c).
Figure SOTERM-32 shows the cumulative distribution function for the humic-acid proportionality constant. All other humic-acid proportionality constants are constant values for both Castile and Salado brines, as shown in Table SOTERM-21.
Variable brine volume in the calculation of radionuclide concentrations in brine was implemented in the CRA-2014 PA. Radionuclide solubilities were calculated in terms of 1×, 2×, 3×, 4×, and 5× the minimum repository brine volume necessary for a DBR. Implementation of multiple brine volumes in PANEL calculations needs actinide solubilities that were calculated over multiple brine volumes by Brush and Domski (Brush and Domski 2013a). The calculated baseline actinide solubilities at 1× and 5× minimum brine volume are listed in Table SOTERM-19. A more detailed discussion of these results and the effects of variable brine volumes can be found in Brush and Domski (Brush and Domski 2013a).
Dissolved and colloidal species may transport differently because of different diffusion rates, sorption onto stationary materials, and size-exclusion effects (filtration and hydrodynamic chromatography). With maximum molecular diffusion coefficients of about 4 ´ 10-10 m2/s, actinides are estimated to diffuse about 10 m in 10,000 years, a negligible distance. Sorption and filtration have beneficial but unquantified effects on performance. Hydrodynamic chromatography may increase colloidal transport over dissolved transport by, at most, a factor of two for theoretically perfect colloidal-transport conditions. In the WIPP, the expected increase is much lower. Given the small or beneficial nature of these effects, they were not included in the CCA PA, the CCA PAVT, the CRA-2004 PA, the CRA-2004 PABC, the CRA-2009 PA, the CRA-2009 PABC, or the CRA-2014 PA calculations of radionuclide transport in the repository.
Figure SOTERM- 32. Cumulative Distribution Function for the Humic-Acid Proportionality Constant for the III Oxidation State in Castile Brine
Because PA does not differentiate dissolved from colloidal species for transport in the Salado, the total source term in the Salado is the sum of both components.. To model transport within the Culebra, however, this simplification was replaced by separating the mobilized actinides delivered to the Culebra by Salado transport codes into five components (dissolved, humic, microbial, mineral-fragment, and intrinsic colloids) to account for differences in their transport behavior. This is important because transport within the repository occurs through, at most, hundreds of meters of poorly defined waste undergoing decomposition, whereas transport through the Culebra occurs over kilometers in a relatively homogeneous (compared to waste) fractured dolomite.
The parameters required to construct the source term were as follows:
Cm and Np are not explicitly transported in NUTS, although they are implicitly lumped with other modeled isotopes. They are, however, included in the PANEL calculations for use with the DBR calculations in PA.
These parameters are combined into a single maximum concentration for each modeled actinide in the PA calculations. The term "total mobilized concentration" is used for the combined concentrations of dissolved and colloidal species. The combined concentrations are not necessarily the actual concentrations, because the concentration may be lower as a result of inventory limits. Both NUTS and PANEL assume that the actinide concentrations specified by the total mobilized concentrations are attained instantaneously as long as sufficient inventory is available. When the inventory is insufficient, the actual mobilized concentration will be lower and is said to be inventory limited. The calculation of the total mobilized concentration is performed by PANEL for each of 100 sampled vectors in a replicate. A similar methodology to generate the combined maximum concentrations was used for the CCA PA, the CCA PAVT, the CRA-2004 PA, the CRA-2004 PABC, the CRA-2009 PA, the CRA-2009 PABC and the CRA-2014 PA.
All of the source term parameters and their associated distributions are entered into the PA parameter database. For each sampled parameter, the Latin Hypercube Sampling code uses the distribution from the PA parameter database to create 100 sampled values. These values are combined with the parameters that have constant values and stored in computational databases for each of the 100 vectors (i.e., 100 realizations), which constitute one replicate. For each realization, PANEL uses both the constant and sampled values for all of the source term parameters, and constructs the source term for NUTS and PANEL, as shown below. This process is repeated for scenarios using the Salado end-member total mobilized concentration and for scenarios using the Castile end-member total mobilized concentration.
Dissolved = Baseline Solubility × 10 Sampled from Solubility Uncertainty Distribution ( SOTERM.74 )
IF (Dissolved × Proportionality Constant of Humic Colloids < Humic Cap),
THEN Humic = Dissolved × Proportionality Constant of Humic Colloid, (SOTERM.75)
Mineral = Database Concentration (a constant value) (SOTERM.76)
Intrinsic = Database Concentration (a constant value) (SOTERM.77)
Microbial_temp = Dissolved × Proportionality Constant of Microbial Colloids,
Total Mobile_temp = Dissolved + Humic + Microbial_temp + Mineral + Intrinsic
IF (Total Mobile_temp < Microbial Cap),
THEN Microbial = Microbial_temp, (SOTERM.78)
ELSE IF( (Dissolved + Humic + Mineral + Intrinsic) > Microbial Cap ),
ELSE Microbial = Microbial Cap - (Dissolved + Humic + Mineral + Intrinsic)
Total Mobile = Dissolved + Humic + Microbial + Mineral + Intrinsic (SOTERM.79)
For actinides with more than one oxidation state, the oxidation state is specified by the oxidation-state parameter
IF (OXSTAT £ 0.5); THEN Lower Oxidation State,
ELSE Higher Oxidation State (SOTERM.80)
where OXSTAT is the oxidation-state parameter sampled from a uniform distribution between 0 and 1.
Solubility calculations are performed for Am(III), Th(IV), and Np(V) and the oxidation-state analogy is used to apply the values calculated for these elements/oxidation states to other actinide elements in the same oxidation states (if any). The total mobilized concentration and mobile fractions for Cm are set equal to the values for Am. In addition, the PA groups radioisotopes with similar decay and transport properties for the NUTS and SECOTP2D (component radionuclide transport in fractures or granular acquifers) transport calculations, as explained in Section SOTERM-5.1.5. For example, the U solubility is decreased to account for the shared solubility with the low-activity 238U, which is not explicitly modeled, enabling NUTS to properly represent the effect of the U isotopes on compliance using the single lumped isotope 234U (Appendix PA-2014, Section PA-4.3.2 ).
PANEL also calculates the fraction of each actinide mobilized by the five different mechanisms, as follows:
Fraction dissolved = Dissolved/Total Mobile (SOTERM.81)
Fraction on humics = Humic/Total Mobile (SOTERM.82)
Fraction in/on microbes = Microbial/Total Mobile (SOTERM.83)
Fraction on mineral fragments = Mineral/Total Mobile (SOTERM.84)
Fraction as intrinsic colloid = Intrinsic/Total Mobile (SOTERM.85)
As an example, for one realization in Salado brine, the sampled value for OXSTAT was 0.9, so Pu would be present in the IV state. The sampled value of the solubility uncertainty distribution was 0.09 for the IV state, which has a median brine solubility of 6.05 ´ 10-8 M. The humic proportionality constant for the IV oxidation state in Salado brine is 6.3, the microbial proportionality constant for Pu is 1.76, the humic cap is 1.1 ´ 10-5 M, the microbe cap for Pu is 2.3 ´ 10-6 M, the concentration of the actinide on mineral fragments is 2.6 ´ 10-8 M, and the Pu intrinsic-colloid concentration is 2 ´ 10-8 M.
For this realization, the maximum dissolved concentration of Pu(IV) used by the PA would be
CPu = (6.05 ´ 10-8) ´ (100.09) = 7.44 ´ 10-8 M. (SOTERM.86)
(The calculations for this example have been rounded to two significant figures, although the PA would not round the intermediate or final values.) CPu is the maximum dissolved concentration of all combined isotopes of Pu.
The maximum humic-complexed Pu would be
(7.44 ´ 10-8 M)(6.3 mol adsorbed per mol) = 4.69 ´ 10-7 M. (SOTERM.87)
This value, however, does not exceed the cap for humic-mobilized Pu, 1.1 ´ 10-5 M. Therefore, in this case, the cap would not be used for the maximum humic-mobilized actinide concentration. Note that the humic-mobilized concentration of Pu exceeds the maximum dissolved concentration of Pu, which is usually the case.
The maximum microbial-mobilized Pu would be
(7.44 ´ 10-8 M)(1.76 mol bioaccumulated per mol) = 1.31 ´ 10-7 M. (SOTERM.88)
This value is less than the cap, 2.3 ´ 10-6 M, so the cap does not affect microbial-mobilized Pu for this realization.
The total mobilized concentration of Pu(IV) for this realization would then be the sum of the dissolved and colloidal contributions (see Equation [SOTERM.79]):
Total Mobile = Dissolved + Humic + Microbial + Mineral + Intrinsic, (SOTERM.89)
= 7.44 ´ 10-8 + 4.69 ´ 10-7 + 1.31 ´ 10-7 + 2.6 ´ 10-8 + 2.0 ´ 10-8,
The output of the PANEL calculations is a computational database containing the source term and effective inventories. NUTS and PANEL both assume instantaneous dissolution and colloidal mobilization up to the solubility limits when sufficient inventory is present, as discussed in Appendix PA-2014, Section PA-4.3.4. Table SOTERM-23 shows the dissolved and colloidal components of the source term and the total mobile actinide concentrations obtained when median parameter values are used. For conservatism, 1× minimum brine volume was used because total mobilized concentration for a radionuclide decreases as the brine volume increases (Kim 2013a).
Table SOTERM- 23. Concentrations (M) of Dissolved, Colloidal, and Total Mobile Actinides Obtained Using Median Parameter Values for the CCA PAVT, CRA-2004 PABC, CRA-2009 PABC and CRA-2014 PAa
|
Actinide Oxidation State and Brine |
PAVT |
CRA-2004 PABC |
CRA-2009 PABC |
CRA-2014 PA |
|
Pu(III), dissolved, Salado brine |
9.75 × 10-8 |
3.61 × 10-7 |
1.96 × 10-6 |
3.46 × 10-7 |
|
Pu(III), colloidal, Salado brine |
7.48 × 10-8 |
2.04 × 10-7 |
9.87 × 10-7 |
7.21 × 10-7 |
|
Pu(III), total mobile, Salado brine |
1.72 × 10-7 |
5.64 × 10-7 |
2.95 × 10-6 |
1.07 × 10-6 |
|
Pu(III), dissolved, Castile brine |
1.06 × 10-8 |
2.68 × 10-7 |
1.78 × 10-6 |
1.98 × 10-7 |
|
Pu(III), colloidal, Castile brine |
4.46 × 10-8 |
4.75 × 10-7 |
3.00 × 10-6 |
6.65 × 10-7 |
|
Pu(III), total mobile, Castile brine |
5.52 × 10-8 |
7.44 × 10-7 |
4.79 × 10-6 |
8.62 × 10-7 |
|
Am(III), dissolved, Salado brine |
9.75 × 10-8 |
3.61 × 10-7 |
1.96 × 10-6 |
3.46 × 10-7 |
|
Am(III), colloidal, Salado brine |
3.96 × 10-7 |
1.39 × 10-6 |
7.45 × 10-6 |
9.57 × 10-8 |
|
Am(III), total mobile, Salado brine |
4.93 × 10-7 |
1.75 × 10-6 |
9.41 × 10-6 |
4.42 × 10-7 |
|
Am(III), dissolved, Castile brine |
1.06 × 10-8 |
2.68 × 10-7 |
1.78 × 10-6 |
1.98 × 10-7 |
|
Am(III), colloidal, Castile brine |
7.78 × 10-8 |
1.34 × 10-6 |
8.88 × 10-6 |
3.01 × 10-7 |
|
Am(III), total mobile, Castile brine |
8.83 × 10-8 |
1.61 × 10-6 |
1.07 × 10-5 |
4.98 × 10-7 |
|
Th(IV), dissolved, Salado brine |
1.06 × 10-8 |
6.70 × 10-8 |
1.70 × 10-8 |
6.46 × 10-7 |
|
Th(IV), colloidal, Salado brine |
1.25 × 10-7 |
6.56 × 10-7 |
1.86 × 10-7 |
4.12 × 10-6 |
|
Th(IV), total mobile, Salado brine |
1.36 × 10-7 |
7.23 × 10-7 |
2.03 × 10-7 |
4.76 × 10-6 |
|
Th(IV), dissolved, Castile brine |
3.33 × 10-8 |
8.07 × 10-8 |
2.11 × 10-8 |
7.50 × 10-7 |
|
Th(IV), colloidal, Castile brine |
3.39 × 10-7 |
7.85 × 10-7 |
2.24 × 10-7 |
4.77 × 10-6 |
|
Th(IV), total mobile, Castile brine |
3.73 × 10-7 |
8.65 × 10-7 |
2.45 × 10-7 |
5.52 × 10-6 |
|
a. Values are calculated using data retrieved from the WIPP PA Database http://tgw.sandia.gov/ and equations SOTERM.74 through SOTERM.79. |
||||
Table SOTERM-23. Concentrations (M) of Dissolved, Colloidal, and Total Mobile Actinides Obtained Using Median Parameter Values for the CCA PAVT, CRA-2004 PABC, CRA-2009 PABC and CRA-2014 PAa (Continued)
|
Actinide Oxidation State and Brine |
PAVT |
CRA-2004 PABC |
CRA-2009 PABC |
CRA-2014 PA |
|
U(IV), dissolved, Salado brine |
1.06 × 10-8 |
6.70 × 10-8 |
1.70 × 10-8 |
6.46 × 10-7 |
|
U(IV), colloidal, Salado brine |
9.26 × 10-8 |
4.48 × 10-7 |
1.33 × 10-7 |
4.13 × 10-6 |
|
U(IV), total mobile, Salado brine |
1.03 × 10-7 |
5.15 × 10-7 |
1.50 × 10-7 |
4.77 × 10-6 |
|
U(IV), dissolved, Castile brine |
3.33 × 10-8 |
8.07 × 10-8 |
2.11 × 10-8 |
7.50 × 10-7 |
|
U(IV), colloidal, Castile brine |
2.36 × 10-7 |
5.35 × 10-7 |
1.59 × 10-7 |
4.78 × 10-6 |
|
U(IV), total mobile, Castile brine |
2.69 × 10-7 |
6.15 × 10-7 |
1.80 × 10-7 |
5.53 × 10-6 |
|
Pu(IV), dissolved, Salado brine |
1.06 × 10-8 |
6.70 × 10-8 |
1.70 × 10-8 |
6.46 × 10-7 |
|
Pu(IV), colloidal, Salado brine |
9.67 × 10-8 |
4.69 × 10-7 |
1.39 × 10-7 |
4.12 × 10-6 |
|
Pu(IV), total mobile, Salado brine |
1.07 × 10-7 |
5.36 × 10-7 |
1.56 × 10-7 |
4.76 × 10-6 |
|
Pu(IV), dissolved, Castile brine |
3.33 × 10-8 |
8.07 × 10-8 |
2.11 × 10-8 |
7.50 × 10-7 |
|
Pu(IV), colloidal, Castile brine |
2.47 × 10-7 |
5.60 × 10-7 |
1.66 × 10-7 |
4.77 × 10-6 |
|
Pu(IV), total mobile, Castile brine |
2.80 × 10-7 |
6.40 × 10-7 |
1.87 × 10-7 |
5.52 × 10-6 |
|
U(VI), dissolved, Salado brine |
7.07 × 10-6 |
1.00 × 10-3 |
1.00 × 10-3 |
1.00 × 10-3 |
|
U(VI), colloidal, Salado brine |
8.89 × 10-7 |
1.31 × 10-5 |
1.31 × 10-5 |
1.11 × 10-5 |
|
U(VI), total mobile, Salado brine |
7.96 × 10-6 |
1.01 × 10-3 |
1.01 × 10-3 |
1.01 × 10-3 |
|
U(VI), dissolved, Castile brine |
7.15 × 10-6 |
1.00 × 10-3 |
1.00 × 10-3 |
1.00 × 10-3 |
|
U(VI), colloidal, Castile brine |
3.69 × 10-6 |
1.31 × 10-5 |
1.31 × 10-5 |
1.11 × 10-5 |
|
U(VI), total mobile, Castile brine |
1.08 × 10-5 |
1.01 × 10-3 |
1.01 × 10-3 |
1.01 × 10-3 |
|
b. Values are calculated using data retrieved from the WIPP PA Database http://tgw.sandia.gov/ and equations SOTERM.74 through SOTERM.79. |
||||
(*Indicates a reference that has not been previously submitted.)
Abdelouas, A., W. Lutze, W. Gong, E.H. Nuttall, B.A. Strietelmeier, and B.J. Travis. 2000. "Biological Reduction of Uranium in Groundwater and Subsurface Soil." The Science of the Total Environment, vol. 250: 21. [Author]
Allard, B. 1982. Solubilities of Actinides in Neutral or Basic Solutions. Actinides in Perspective. N. Edelstein, ed. New York: Pergamon. pp. 553-80. [Author]
Allard, S., and C. Ekberg. 2006a. "Complexing properties of a α-isosaccharinate: thorium." Radiochimica Acta 94, 537-540.* [Author]
Allard, S., and C. Ekberg. 2006b. "Complexing properties of α-isosaccharinate: stability constants, enthalpies and entropies of Th-complexation with uncertainty analysis." J. Solution Chem. 35, 1173-1186.* [Author]
Altmaier, M., V. Metz, V. Neck, R. Muller, and T. Fanghänel. 2003. "Solid-Liquid Equilibria of Mg(OH)2(cr) and Mg2(OH)3Cl4H2O(cr) in the System Mg-Na-H-OH-Cl-H2O at 25ºC." Geochimica et Cosmochimica Acta, vol. 67: 3595-3601. [Author]
Altmaier, M., V. Neck, and Th. Fanghänel. 2004. "Solubility and Colloid formation of Th(IV) in concentrated NaCl and MgCl2 Solutions." Radiochimica Acta, vol. 92: 537-43. [Author]
Altmaier, M., V. Neck, and Th. Fanghänel. 2008. "Solubility of Zr(IV), Th(IV) and Pu(IV) hydrous oxides in CaCl2 solutions and the formation of ternary Ca-M(IV)-OH complexes." Radiochimica Acta vol. 96: (9-11) 541-550.* [Author]
Altmaier, M., V. Neck, M.A. Denecke, R. Yin, and Th. Fanghänel. 2006. "Solubility of ThO2 ·xH2O(am) and the Formation of Ternary Th(IV) Hydroxide-Carbonate Complexes in NaHCO3-Na2CO3 Solutions Containing 0-4M NaCl." Radiochimica Acta, vol. 94: 495-500. [Author]
Altmaier, M., V. Neck, R. Müller, and Th. Fanghänel. 2005. "Solubility of ThO2 xH2O(am) in Carbonate Solution and the Formation of Ternary Th(IV) Hydroxide-carbonate Complexes." Radiochimica Acta, vol. 93: 83-92. [Author]
Ams, D.A., J.S. Swanson, J. Szymanowski, J.B. Fein, M. Richmann, and D.T. Reed. 2013. "The Effect of High Ionic Strength on Neptunium (V) Adsorption to a halophilic bacterium." Geochimica et Comoschimica Acta, vol.: 110: 45-57.* [Author]
Banaszak, J.E., D.T. Reed, and B.E. Rittmann. 1998a. "Speciation-Dependent Toxicity of Neptunium(V) Towards Chelatobacter heintzii." Environmental Science and Technology, vol. 32: 1085-91. [Author]
Banaszak, J.E., B.E. Rittmann, and D.T. Reed. 1998b. Subsurface Interactions of Actinide Species and Microorganisms: Implications for the Bioremediation of Actinide-Organic Mixtures. ANL-98/26. Argonne, IL: Argonne National Laboratory. [Author]
Banaszak, J.E., S.M. Webb, B.E. Rittmann, J.-F. Gaillard, and D.T. Reed. 1999. "Fate of Neptunium in Anaerobic, Methanogenic Microcosm." Scientific Basis for Nuclear Waste Management XXII, vol. 556: 1141-49. [Author]
Barnhart, B.J., E.W. Campbell, J.M. Hardin, E. Martinez, D.E. Caldwell, and R. Hallett. 1978a. Potential microbial impact on transuranic wastes under conditions expected in the Waste Isolation Pilot Plant (WIPP). LA-7788-PR Progress Report, October-December 15, 1978. Los Alamos, NM: Los Alamos Scientific Laboratory.* [PDF / Author]
Barnhart, B.J., E.W. Campbell, J.M. Hardin, E. Martinez, D.E. Caldwell, and R. Hallett. 1979b. Potential microbial impact on transuranic wastes under conditions expected in the Waste Isolation Pilot Plant (WIPP). LA-7839-PR Progress Report, December 15, 1978-March 15, 1979. Los Alamos, NM: Los Alamos Scientific Laboratory.* [PDF / Author]
Barnhart, B.J., E.W. Campbell, E. Martinez, D.E. Caldwell, and R. Hallett. 1979c. Potential microbial impact on transuranic wastes under conditions expected in the Waste Isolation Pilot Plant (WIPP). LA-7918-PR Progress Report, March 15-June 15, 1979. Los Alamos, NM: Los Alamos Scientific Laboratory.* [PDF / Author]
Barnhart, B.J., E.W. Campbell, J.M. Hardin, E. Martinez, D.E. Caldwell, and R. Hallett. 1979d. Potential microbial impact on transuranic wastes under conditions expected in the Waste Isolation Pilot Plant (WIPP). LA-8297-PR Progress Report, October 1, 1978-September 30, 1979. Los Alamos, NM: Los Alamos Scientific Laboratory.* [PDF / Author]
Barton, L.L., K. Choudhury, B.M. Thomson, K. Steenhoudt, and A.R. Groffman. 1996. "Bacterial Reduction of Soluble Uranium: The First Step of In Situ Immobilization of Uranium." Radioactive Waste Management and Environmental Restoration, vol. 20: 141-51. [Author]
Behrends, T., and P. Van Cappellen. 2005. "Competition Between Enzymatic and Abiotic Reduction of Uranium(VI) under Iron Reducing Conditions." Chemical Geology, vol. 220: 315-27. [Author]
Bender, J., M.C. Duff, P. Phillips, and M. Hill. 2000. "Bioremediation and Bioreduction of Dissolved U(VI) by Microbial Mat Consortium Supported on Silica Gel Particles." Environmental Science and Technology, vol. 34: 3235-41. [Author]
Bennett, D., Y. Wang, and T. Hicks. 1996. Memorandum to Distribution (Subject: An Evaluation of Heat Generation Processes for the WIPP). 20 August 1996. ERMS 240635. Albuquerque, NM: Sandia National Laboratories.* [PDF / Author]
Birbir, M., B. Calli, B. Mertoglu, R. Bardavid, A. Oren, M. Ogmen and A. Ogan. 2007. "Extremely Halophilic Archaea from Tuz Lake, Turkey, and the Adjacent Kaldirim and Kayacik Salterns." World J. Microbiol Biotechnol, 23:309-316.* [Author]
Borkowski, M., J.-F. Lucchini, M.K. Richmann, and D.T. Reed. 2008. Actinide (III) Solubility in WIPP Brine: Data Summary and Recommendations. LCO-ACP-08, LANL\ACRSP Report, LA-14360. Los Alamos, NM: Los Alamos National Laboratory. [PDF / Author]
Borkowski, M. J-F. Lucchini, M.K. Richmann, and D.T. Reed. 2010a. Actinide (III) Solubility in WIPP Brine: Data Summary and Recommendations. Report LA-14360. Los Alamos, NM: Los Alamos National Laboratory.* [PDF / Author]
Borkowski, M., M.K. Richmann, D.T. Reed, and Y.-L. Xiong. 2010b. "Complexation of Nd(III) with Tetraborate Ion and Its Effect on Actinide(III) Solubility in WIPP Brine." Radiochimica Acta, vol.: 98.9-11 (2010): 577-582.* [Author]
Borkowski, M., M.K. Richmann, and J-F. Lucchini. 2012. Solubility of An(IV) in WIPP Brine: Thorium Analog Studies in WIPP Simulated Brine. Los Alamos Report LCO-ACP-17, LA-UR 12-24417. Carlsbad, NM: Los Alamos National Laboratory.* [PDF / Author]
Brendebach, B., M. Altmaier, J. Rothe, V. Neck, and M.A. Denecke. 2007. EXAFS Study of Aqueous Zr(IV) and Th(IV) Complexes in Alkaline CaCl2 Solutions: Ca3[Zr(OH)6]4+ and Ca4[Th(OH)8] 4+." Inorganic Chemistry, vol. 46: 6804-10. [Author]
Brush, L.H. 1990. Test Plan for Laboratory and Modeling Studies of Repository and Radionuclide Chemistry for the Waste Isolation Pilot Plant. SAND90-0266. ERMS 226015. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Brush, L.H. 1995. Systems Prioritization Method-Iteration 2 Baseline Position Paper: Gas Generation in the Waste Isolation Pilot Plant (March 17). ERMS 228740. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Brush, L.H., and J. Garner. 2005. Letter to D. Kessel (Subject: Additional Justification for the Insignificant Effect of Np on the Long-Term Performance of the WIPP). 21 February 2005. ERMS 538533. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Brush, L.H., and P.S. Domski. 2013a. Prediction of Baseline Actinide Solubilities for the WIPP CRA-2014 PA. Analysis report, January 21, 2013. ERMS 559138. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Brush, L.H., and P.S. Domski. 2013b. Calculation of Organic-Ligand Concentrations for the WIPP CRA-2014 PA. Analysis report, January 14, 2013. ERMS 559005. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Brush, L.H., and P.S. Domski. 2013c. Uncertainty Analysis of Actinide Solubilities for the WIPP CRA-2014 PA, Rev. 1 Supercedes ERMS 559278. ERMS 559712. Albuquerque, NM: Sandia National Laboratories.* [PDF / Author]
Brush, L.H., and Y. Xiong. 2005a. Calculation of Organic-Ligand Concentrations for the WIPP Performance Assessment Baseline Calculations (May 4). ERMS 539635. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Brush, L.H., and Y. Xiong. 2005b. Calculation of Actinide Solubilities for the WIPP Performance Assessment Baseline Calculations, Analysis AP-120, Rev. 0 (Rev. 0). AP-120. ERMS 539255. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Brush, L.H., P.S. Domski, and Y.-L. Xiong. 2011 Predictions of the Compositions of Standard WIPP Brines as a Function of pcH for Laboratory Studies of the Speciation and Solubilities of Actinides. Analysis report, June 23, 2011. ERMS 555727 Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Brush, L.H., R.C. Moore, and N.A. Wall. 2001. Response to EEG-77, Plutonium Chemistry under Conditions Relevant for WIPP Performance Assessment: Review of Experimental Results and Recommendations for Future Work, by V. Oversby. ERMS 517373. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Bundschuh, T., R. Knopp, R. Müller, J.I. Kim, V. Neck, and Th. Fanghänel. 2000. "Application of LIBD to the Determination of the Solubility Product of Thorium(IV)-Colloids." Radiochimica Acta, vol. 88: 625-629. [Author]
Büppelmann, K., J.I. Kim, and Ch. Lierse. 1988. "The Redox Behavior of Pu in Saline Solutions under Radiolysis Effects." Radiochimica Acta, vol. 44/45: 65-70. [Author]
Büppelmann, K., S. Magirius, Ch. Lierse, and J.I. Kim. 1986. "Radiolytic Oxidation of Am (III) to Am (V) and Pu(IV) to Pu (VI) in Saline Solution." Journal of Less- Common Metals, vol. 122: 329-36. [Author]
Caldwell, D., R. Hallet, M. Molecke, E. Martinez, and B. Barnhart. 1988. Rates of CO2 Production From the Microbial Degradation of Transuranic Wastes Under Simulated Geologic Isolation Conditions. Report SAND87-7170. Carlsbad NM: Sandia National Laboratories.* [PDF / Author]
Camphouse, R.C., D.C. Kicker, S. Kim, T. Kirchner, J. Long, B. Malama, and T. Zeitler. 2013. Summary Report for the 2014 WIPP Compliance Recertification Application Performance Assessment. ERMS 560252. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Casas, I., J. De Pablo, J. Gimenez, M.E. Torrero, J. Bruno, E. Cera, R.J. Finch, and R.C. Ewing. 1998. "The Role of pe, pH, and Carbonate on the Solubility of UO2 and Uraninite under Nominally Reducing Conditions." Geochimica et Cosmochimica Acta, vol. 62: 2223-31. [Author]
Chen, T., Wang, X., Tian, W., Sun, M., Li, C., Liu, X., Wang, L., and C. Liu. 2010. "Solubility Analysis of Americium in Yuci Groundwater." Acta Physico-Chimica Sinica, vol. 26(4), 811-816.* [Author]
Choppin, G.R. 1988. Letter to L.H. Brush (Subject: Chemicals Listed Could Interact with Actinides). 29 December 1988. Tallahassee, FL: WIPP Central Files. [PDF / Author]
Choppin, G.R. 1999. "Utility of Oxidation State Analogs in the Study of Plutonium Behavior." Radiochimica Acta, vol. 85: 89-95. [Author]
Choppin, G.R., A.H. Bond, M. Borkowski, M.G. Bronikowski, J.G. Chen, S. Lis, J. Mizera, O. Pokrovski, N.A. Wall, Y.X. Xia, and R.C. Moore. 1999. WIPP Actinide Source Term Test Program: Solubility Studies and Development of Modeling Parameters. SAND 99-0943. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Choppin, G.R., and L.F. Rao. 1992. Reduction of Neptunium(VI) by Organic Compounds. Transuranium Elements: A Half Century (pp. 262-75). L.R. Morss and J. Fuger, eds. Washington, DC: American Chemical Society. [Author]
Choppin, G.R., J. Liljenzin, and J.O. Rydberg. 2004. Radiochemistry and Nuclear Chemistry. 3rd ed. Woburn, MA: Butterworth-Heinenmann. [Author]
Christensen, T.H., P.L. Bjerg, S.A. Banwart, R. Jakobsen, G. Heron, and H-J. Albrechtsen. 2000. "Characterization of Redox Conditions in Groundwater Contaminant Plumes." Journal of Contaminant Hydrology, vol. 45: 165-241. [Author]
Clark, D.L., and C.D. Tait. 1996. Memorandum to Sandia WIPP Records Center (Subject: SWCF-A: 1.1.10.1.1: NQ: Actinide Source Term: LANL Monthly Reports). Sandia WIPP Central File A: WBS 1.1.10.1.1. WPO 31106. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Clark, D.L., D.E. Hobart, and M.P. Neu. 1995. "Actinide Carbonate Complexes and Their Importance in Actinide Environmental Chemistry." Chemical Reviews, vol. 95: 25.* [Author]
Clayton, D.J. 2008. Memorandum to Larry Brush (Subject: Update to the Calculation of the Minimum Brine Volume for a Direct Brine Release). 2 April 2008. ERMS 548522. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Clayton, D.J. 2013. Justification of Chemistry Parameters for Use in BRAGFLO for AP-164, Revision 1. ERMS 559466. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Clayton, D.J., R.C. Camphouse, J.W. Garner, A.E. Ismail, T.B. Kirchner, K.L. Kuhlman, and M.B. Nemer. 2010. Summary Report of the CRA-2009 Performance Assessment Baseline Calculation. ERMS 553039. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Cleveland, J.M. 1979. The Chemistry of Plutonium. La Grange Park, IL: American Nuclear Society. [Author]
Cotton, F.A., and G. Wilkinson. 1988. Advanced Inorganic Chemistry. 5th ed. New York: Wiley. [Author]
Cronin, E., and F. Post. 1977. "Report of a Dematiaceous Hyphomycete From the Great Salt lake, Utah." Mycologia, 69: 846-847.* [Author]
Cui, D., and K. Spahiu. 2002. "The Reduction of U(VI) on Corroded Iron under Anoxic Conditions." Radiochimica Acta, vol. 90: 623-28. [Author]
David, F., A.G. Maslennikov, and V.P. Peretrukhin. 1990. "Electrochemical Reduction of Actinides ions in Aqueous solution: Application to Separations and Some Intermetallic Compound Synthesis." Journal of Radioanalytical Nuclear Chemistry, vol. 143: 415-26. [Author]
Degueldre, C., and A. Kline. 2007. "Study of Thorium Association and Surface Precipitation on Colloids." Earth and Planetary Science Letters, vol. 264: 104-13. [Author]
Deng, H., S. Johnson, Y. Xiong, G.T. Roselle, and M. Nemer. 2006. Analysis of Martin Marietta MagChem 10 WTS-60 MgO. ERMS 544712. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Diaz-Arocas, P., and B. Grambow. 1998. "Solid-liquid Phase Equilibria of U(VI) in NaCl Solutions." Geochimica et Cosmochimica Acta, vol. 62: 245-63. [Author]
Dodge, C.J., A.J. Francis, J.B. Gillow, G.P. Halada, C. Eng, and C.R. Clayton. 2002. "Association of Uranium with Iron Oxides Typically Formed on Corroding Steel Surfaces." Environmental Science and Technology, vol. 36: 3504-11. [Author]
Draganic, I.G., and Z.D. Draganic. 1971. Primary Products of Water Radiolysis: Oxidizing Species-the Hydroxyl Radical and Hydrogen Peroxide. The Radiation Chemistry of Water (pp. 91-121). New York: Academic. [Author]
Drez, P.E. 1991. Preliminary Nonradionuclide Inventory of CH-TRU Waste, Preliminary Comparison with 40 CFR Part 191, Subpart B for the Waste Isolation Pilot Plant, December 1991. Volume 3: Reference Data (pp. A-43 through A-53). Eds. R.P. Rechard, A.C. Peterson, J.D. Schreiber, H.J. Iuzzolino, M.S. Tierney, and J.S. Sandha. SAND91-0893/3. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Du, X., B. Boonchayaanant, W.-M. Wu, S. Fendorf, J. Bargar, and C.S. Criddle. 2011. "Reduction of Uranium(VI) by Soluble Iron(II) Conforms with Thermodynamic Predictions." Environmental Science & Technology, vol.45: 4718-25.* [Author]
Ekberg, C., Y. Albinsson, M.J. Comarmond, and P.L. Brown. 2000. "Studies on the Behavior of Thorium(IV)." Journal of Solution Chemistry, vol. 29, no. 1: 63-86. [Author]
Emmerich, M., A Bhansali, T. Lösekann-Behrens, C. Schröder, A. Kappler, and S. Behrens. 2012. "Abundance, Distribution, and Activity of Fe(II)-Oxidizing and Fe(III)-Reducing Microorginaism in Hypersaline Sediments of Lake Kasin, Southern Russia." Appl. Environ. Microbiol, 78(12): 4386-4399.* [Author]
Eriksen, T.E., P. Ndamamba, D. Cui, J. Bruno, M. Caceci, and K. Spahiu. 1993. SKB Technical Report 93-18. Stockholm: Svensk Kärnbränsleforsorjning AB. [Author]
Ershov, B.G., M. Kelm, E. Janata, A.V. Gordeev, and E. Bohnert. 2002. "Radiation-Chemical Effects in the Near-Field of a Final Disposal Site: Role of Bromine on the Radiolytic Processes in NaCl-Solutions." Radiochimica Acta, vol. 90: 617. [Author]
Fanghänel, Th., and J.I. Kim. 1998. "Spectoscopic Evaluation of Thermodynamics of Trivalent Actinides in Brines." Journal of Alloys and Compounds, vol. 271-273: 728-737. [Author]
Fanghänel, Th., and V. Neck. 2002. "Aquatic Chemistry and Solubility Phenomena of Actinide Oxides/hydroxides." Pure Applied Chemistry, vol. 74: 1895-1907. [Author]
Fanghänel, Th., V. Neck, and J.I. Kim. 1995. "Thermodynamics of Neptunium(V) in Concentrated Salt Solutions: II. Ion Interaction (Pitzer) Parameters for Np(V) Hydrolysis Species and Carbonate Complexes." Radiochimica Acta, vol. 69: 169-76. [Author]
Farrell, J., W.D. Bostick, R.J. Jarabeck, and J.N. Fiedor. 1999. "Uranium Removal from Ground Water Using Zero Valent Iron Media." Ground Water, vol. 37: 618-24. [Author]
Felmy, A.R., D. Rai, and R.W. Fulton. 1990. "The Solubility of AmOHCO3(cr) and the Aqueous Thermodynamics of the System Na+-Am3+-HCO3--CO32--OH-H2O." Radiochimica Acta, vol. 50: 193-204. [Author]
Felmy, A.R., D. Rai, J.AS. Schramke, and J.L. Ryan. 1989. "The Solubility of Plutonium Hydroxide in Dilute Solution and in High-Ionic Strength Chloride Brines." Radiochimica Acta, vol. 48: 29-35. [Author]
Felmy, A.R., D. Rai, S.M. Sterner, M.J. Mason, N.J. Hess, and S.D. Conradson. 1996. Thermodynamic Models for Highly Charged Aqueous Species: The Solubility of Th(IV) Hydrous Oxide in Concentrated NaHCO3 and Na2CO3 Solutions (August 14). ERMS 240226. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Felmy, A.R., M.J. Mason, and D. Rai. 1991. "The Solubility of Hydrous Thorium(IV) Oxide in Chloride Media: Development of an Aqueous Ion-Interaction Model." Radiochimica Acta, vol. 55: 177-85. [Author]
Fiedor, J.N., W.D. Bostick, R.J. Jarabek, and J. Farrell. 1998. "Understanding the Mechanism of Uranium Removal from Groundwater by Zero-Valent Iron Using X-Ray Photoelectron Spectroscopy." Environmental Science and Technology, vol. 32: 1466-73. [Author]
Field, E., S. D'Imperio, A. Miller, M. VanEngelen, R. Gerlach, B. Lee, W. Apel, and B. Peyton. 2010. "Application of Molecular Techniques To Elucidate the Influence of Cellulosic Waste on the Bacterial Community Structure at a Simulated Low-Level-Radioactive-Waste Site." Appl. Environ. Microbiol., 76(10): 3106-3115.* [Author]
Francis, A.J. 1998. "Biotransformation of Uranium and Other Actinides in Radioactive Wastes." Journal of Alloys and Compounds, vol. 271-273: 78-84. [Author]
Francis, A.J., and J.B. Gillow. 1994. Effects of Microbial Processes on Gas Generation Under Expected Waste Isolation Pilot Plant Repository Conditions. Progress Report through 1992. SAND93-7036. WPO 26555. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Francis, A.J., C.J. Dodge, and J. B. Gillow. 2008. "Reductive Dissolution of Pu(IV) by Costridium sp. under Anaerobic Conditions." Environmental Science and Technology, vol. 42: 2355-60. [Author]
Francis, A.J., C.J. Dodge, J.B. Gillow, and H.W. Papenguth. 2000. "Biotransformation of Uranium Compounds in High Ionic Strength Brine by a Halophilic Bacterium under Denitrifying Conditions." Environmental Science and Technology, vol. 34: 2311. [Author]
Francis, A.J., J.B. Gillow, and M.R. Giles. 1997. Microbial gas generation under expected Waste Isolation Pilot Plant repository conditions. SAND96-2582 Report. Albuquerque, NM: Sandia National Laboratories.* [PDF / Author]
Francis, A.J., J.B. Gillow, C.J. Dodge, R. Harris, T.J. Beveridge, and H.W. Papenguth. 2004. "Uranium Association with Halophilic and non-Halophilic Bacteria and Archaea." Radiochimica Acta, vol. 92: 481-88.* [Author]
Fredrickson, J.K., J.M. Zachara, D.W. Kennedy, M.C. Duff, Y.A. Gorby, S.W. Li, and K.M. Krupka. 2000. "Reduction of U(VI) in Goethite (α-FeOOH) Suspensions by a Dissimilatory Metal-Reducing Bacteria." Geochimica et. Cosmochimica Acta, vol. 64: 3085-98. [Author]
Garner, J.W. 1996. Radioisotopes to be Used in the 1996 CCA Calculations. ERMS 540572. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Garner, J.W., and C. Leigh. 2005. Analysis Package for PANEL: CRA-2004 Performance Assessment Baseline Calculation (Revision 0). ERMS 540572. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Gayer, K.H., and H. Leider. 1957. "The Solubility of Uranium (IV) Hydroxide in Solutions of Sodium Hydroxide and Perchloric Acid at 25°C." Canadian Journal of Chemistry, vol. 35: 5-7. [Author]
Giambalvo, E.R. 2002a. Memorandum to L.H. Brush (Subject: Recommended Parameter Values for Modeling An(III) Solubility in WIPP Brines). 25 July 2002. ERMS 522982. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Giambalvo, E.R. 2002b. Memorandum to L.H. Brush (Subject: Recommended Parameter Values for Modeling An(IV) Solubility in WIPP Brines). 26 July 2002. ERMS 522986. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Giambalvo, E.R. 2002c. Memorandum to L.H. Brush (Subject: Recommended Parameter Values for Modeling An(V) Solubility in WIPP Brines). 26 July 2002. ERMS 522990. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Gillow, J.B., M. Dunn, A.J. Francis, D. A. Lucero, and H.W. Papenguth. 2000. "The Potential of Subterranean Microbes in Facilitating Actinide Migration at the Grimsel Test Site and Waste Isolation Pilot Plant." Radiochimica Acta, vol. 88: 769-74. [Author]
Gillow J.B.,and A.J. Francis. 2006. Microbial gas generation under expected Waste Isolation Pilot Plant Repository Conditions: Final Report. Report BNL-96148-2011-IR. Albuquerque, NM: Sandia National Laboratories.* [PDF / Author]
Grambow, B., E. Smailos, H. Geckeis, R. Muller, and H. Hentschel. 1996. "Sorption and Reduction of Uranium (VI) on Iron Corrosion Products under Reducing Saline Conditions." Radiochimica Acta, vol. 74: 149-54. [Author]
Grenthe, I., D. Ferri, F. Salvatore, and G. Riccio. 1984. "Studies on Metal Carbonate Equilibria. Part 10. A Solubility Study of the Complex Formation in the Uranium (VI)-Water-Carbon Dioxide (g) System at 25ºC." Journal of Chemical Society, Dalton Trans.: 2439-43. [Author]
Griffith, J., S. Wilcox, D. Powers, R. Nelson, and B. Baxter. 2008. "Discovery of Abundant Cellulose Microfibers Encased in 250 Ma Permian Halite: A Macromolecular Target in the Search for Life on Other Planet." Astrobiology, 8(2): 215-228.* [Author]
Gu, B., L. Liang, M.J. Dickey, X. Yin, and S. Dai. 1998. "Reductive Precipitation of Uranium (VI) by Zero-Valent Iron." Environmental Science and Technology, vol. 32: 3366-73. [Author]
Guillaumont, R., T. Fanghänel, J. Fuger, I. Grenthe, V. Neck, D.A. Palmer, and M.H. Rand. 2003. "Update on the Chemical Thermodynamics of Uranium, Neptunium, Plutonium, Americium and Technetium." F.I. Mompean, M. Illemassene, C. Domenech-Orti, and K. Ben Said, eds. Chemical Thermodynamics. Vol. 5. Amsterdam: Elsevier. [Author]
Gunde-Cimerman , N., J. Ramos, and A. Plemenitas. 2009. "Halotolerant and Halophilic Fungi." Mycological Research, 113: 1231-1241.* [Author]
Harvie, C.E., N. Møller, and J.H. Weare. 1984. "The Prediction of Mineral Solubilities in Natural Waters, The Na-K-Mg-Ca-H-Cl-SO4-OH-HCO3-CO2-H2O System to High Ionic Strengths at 25°C." Geochimica et Cosmochimica Acta, vol. 48: 723-51. [Author]
Haschke, J.M., and T.E. Ricketts. 1995. Plutonium Dioxide Storage: Conditions for Preparing and Handling. LA-12999. Los Alamos, NM: Los Alamos National Laboratory. [PDF / Author]
Haschke, J.M., T.H. Allen, and L.A. Morales. 2000. "Reaction of Plutonium Dioxide with Water: Formation and Properties of PuO2+x." Science, vol. 287: 285-87. [Author]
Hobart, D.E. 1990. Actinides in the Environment. Proceedings of the Robert A. Welch Foundation Conference on Chemical Research, No. XXXIV: 50 Years With Transuranium Elements (pp. 378-436). Houston, TX: Robert A. Welch Foundation. [Author]
Hobart, D.E., and R.C. Moore. 1996. Analysis of Uranium (VI) Solubility Data for WIPP Performance Assessment. (May 28, 1996). AP-028. Unpublished report. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Hobart, D.E., K. Samhoun, and J. R. Peterson. 1982. "Spectroelectrochemical Studies of the Actinides: Stabilization of Americium (IV) in Aqueous Carbonate Solution." Radiochimica Acta, vol. 31: 139-45. [Author]
Holm, T.R., and C.D. Curtiss. 1989. "A Comparison of Oxidation-Reduction Potentials Calculated from the As(V)/As(III) and Fe(III)/Fe(II) Couples with Measured Platinum-Electrode Potentials in Groundwater." Journal of Contaminant Hydrology, vol. 5: 67-81.* [Author]
Huang, F.Y C., P.V. Brady, E.R. Lindgren, and P. Guerra. 1998. "Biodegradation of Uranium-Citrate Complexes: Implications for Extraction of Uranium from Soils." Environmental Science and Technology, vol. 32: 379. [Author]
Icopini, G.A., J. Boukhalfa, and M.P. Neu. 2007. "Biological Reduction of Np(V) and Np(V) Citrate by Metal-Reducing Bacteria." Environmental, Science, & Technology, vol. 41: 2764-69. [Author]
Ilton, E.S., J.S. Lezama Pacheco, J.R. Bargar, Z. Shi, J. Liu, L. Kovarik, M.H. Engelhard, and A.R. Felmy. 2012. "Reduction of U(VI) Incorporated in the Structure of Hematite." Environmental Science & Technology, vol.46: 9428-36.* [Author]
Ionova, G., C. Madic, and R. Guillamount. 1998. "About the Existence of Th(III) in Aqueous Solution." Polyhedron, vol. 17: 1991-95. [Author]
Ismail, A.E. 2007 Memorandum to File (Subject: Revised Porosity Estimates for the DRZ). 10 April 2007. ERMS 545755. Carlsbad NM: Sandia National Laboratories. [PDF / Author]
Itagaki, H., S. Nakayama, S. Tanaka, and M. Yamawaki. 1992. "Effect of Ionic Strength on the Solubility of Neptunium(V) Hydroxide." Radiochimica Acta, vol. 58/59: 61-66. [Author]
Katz, J.J., G.T. Seaborg, and L.R. Morss. 1986. The Chemistry of the Actinide Elements. 2nd ed. New York: Chapman and Hall. [Author]
Keller, C. 1971. The Chemistry of Transuranium Elements. Weinheim, Germany: Verlag Chemie. [Author]
Kelm, M., I. Pashalidis, and J.I. Kim. 1999. "Spectroscopic Investigation on the Formation of Hypochlorite by Alpha Radiolysis in Concentrated NaCl Solutions." Applied Radiation and Isotopes, vol. 51: 637-42. [Author]
Kerber Schütz, M., N. Lopes, A. Cenci, R. Ligabue, J. Dullius, S. Einloft, and J.M. Ketzer. 2011. "Effect of Time on the Carbonation Reaction of Saline Aquifers with Controlled pH." Energy Procedia, vol.4 :4546-51.* [Author]
Kerisit, S., A.R. Felmy, and E.S. Ilton. 2011. "Influence of Magnetite Stoichiometry on UVI Reduction." Environmental Science & Technology, vol.45: 2770-76.* [Author]
Khasanova, A.B., N.S. Shcherbina, S.N. Kalmykov, Yu.A. Teterin, and A.P. Novikov. 2007. "Sorption of Np(V), Pu(V), and Pu(VI) on Colloids of Fe(III) Oxides and Hydrous Oxides and MnO2." Radiochemistry, vol. 49: 419-25. [Author]
Kicker, D.C. and T. Zeitler. 2013. Radionuclide Inventory Screening Analysis for the 2014 Compliance Recertification Application Performance Assessment (CRA-2014 PA). ERMS 559257. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Kim, J.I., Ch. Apostolidis, G. Buckau, K. Buppelmann, B. Kanellakopulos, Ch. Lierse, S. Magirius, R. Stumpe, I. Hedler, Ch. Rahner, and W. Stoewer. 1985. Chemisches Verhalten von Np, Pu und Am in verschiedenen konzentrierten Salzloesunger = Chemical Behaviour of Np, Pu, and Am in Various Brine Solutions. RCM 01085. Munich, Germany: Institut für Radiochemie der Technische Universitaet Muenchen. (Available from National Technical Information Service, 555 Port Royal Road, Springfield, VA 22161, 703/487-4650 as DE857 2334.) [Author]
Kim, J.I., M. Bernkopf, Ch. Lierse, and F. Koppold. 1984. Hydrolysis Reactions of Am(III) and Pu(VI) Ions in Near-Neutral Solutions. Geochemical Behavior of Disposed Radioactive Waste (pp. 115-34). G.S. Barney, J.D. Navratill, and W.W. Schultz, eds. ACS Symposium Series No. 246. Washington, DC: American Chemical Society. [Author]
Kim, S. 2013a. Analysis Package for Salado Transport Calculations: CRA-2014 Performance Assessment. ERMS 560174. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Kim, S. 2013b. Analysis Package for PANEL: CRA-2014 Performance Assessment. ERMS 560056. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Kim, S.S., M.H. Baik, J.W. Choi, H.S. Shin, and J.I. Yun. 2010. "The dissolution of ThO2(cr) in carbonate solutions and a granitic groundwater." J. Radioanal. Nucl. Chem., 286: 91-97. [Author]
Kim, W.H., K.C. Choi, K.K. Park, and T.Y. Eom. 1994. "Effects of Hypochlorite Ion on the Solubility of Amorphous Schoepite at 25°C in Neutral to Alkaline Aqueous Solutions." Radiochimica Acta, vol. 66/67: 45-49. [Author]
Klapötke, T.M., and A. Schulz. 1997. "The First Observation of a Th3+ ion in Aqueous Solution." Polyhedron, vol. 16: 989-91. [Author]
Korpusov, G.V., E.N. Patrusheva, and M.S. Dolidze. 1975. "The Study of Extraction Systems and the Method of Separation of Trivalent Transuranium Elements Cm, Bk, and Cf." Soviet Radiochemistry, vol. 17: 230-36. [Author]
Kramer-Schnabel, U., H. Bischoff, R.H. Xi, and G. Marx. 1992. "Solubility Products and Complex Formation Equilibria in the Systems Uranyl Hydroxide and Uranyl Carbonate at 25°C and I=0.1M." Radiochimica Acta, vol. 56: 183-88. [Author]
Langmuir, D., and J.S. Herman. 1980. "Mobility of Thorium in Natural Waters at Low Temperatures." Geochimica et. Cosmochimica Acta, vol. 44: 1753-66. [Author]
Latta, D.E., C.A. Gorski, M.I. Boyanov, E.J. O'Loughlin, K.M. Kemner, and M.M. Scherer. 2012. "Influence of Magnetite Stoichiometry on UVI Reduction." Environmental Science & Technology, vol.46: 778-86.* [Author]
LaVerne, J.A., and L. Tandon. 2002. "H2 Production in the Radiolysis of Water on CeO2 and ZrO2." Journal of Physical Chemistry B, vol. 106: 380-86. [Author]
Leigh, C., J. Kanney, L. Brush, J. Garner, R. Kirkes, T. Lowry, M. Nemer, J. Stein, E. Vugrin, S. Wagner, and T. Kirchner. 2005. 2004 Compliance Recertification Application Performance Assessment Baseline Calculation (Revision 0). ERMS 541521. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Lemire, R.J., J. Fuger, H. Nitsche, P. Potter, M.H. Rand, J. Rydberg, K. Spahiu, J.C. Sullivan, W.J. Ullman, P. Vitorge, and H. Wanner. 2001. Chemical Thermodynamics of Neptunium and Plutonium. Amsterdam: Elsevier. [Author]
Lin, M.R., P. Paviet-Hartmann, Y. Xu, and W.H. Runde. 1998. Uranyl Compounds in NaCl Solutions: Structure, Solubility and Thermodynamics. 216th ACS National Meeting: Preprints of Extended Abstracts, vol. 38: 208. Abstract for the National Meeting of the American Chemical Society, Division of Environmental Chemistry, Boston: 23-27 August. [Author]
Lloyd JR, and L.E. Macaskie. 2002. Biochemical basis of microbe-radionuclide interactions. In: Keith-Roach MJ, Livens FR, editors. Interactions of Microorganisms with Radionuclides. London: Elsevier Science Ltd. pp. 313-342.* [Author]
Lloyd, J.R., P. Yong, and L.E. Macaskie. 2000. "Biological Reduction and Removal of Np(V) by Two Microorganisms." Environmental Science and Technology, vol. 34: 1297-1301. [Author]
Lovley, D.R., E.E. Roden, E.J.P. Phillips, and J.C. Woodward. 1993. "Enzymatic Iron and Uranium Reduction by Sulfate-Reducing Bacteria." Marine Geology, vol. 113: 41. [Author]
Lovley, D.R., E.J.P. Phillips, Y.A. Gorbi, and E.R. Landa. 1991. "Microbial Reduction of Uranium." Nature, vol. 350: 413. [Author]
Lucchini, J.-F., H. Khaing, M. Borkowski, M.K. Richmann, and D.T. Reed. 2010a. Actinide (VI) Solubility in Carbonate-free WIPP Brine: Data Summary and Recommendations. LCO-ACP-10, LANL\ACRSP Report. LA-UR 10-00497. Los Alamos, NM: Los Alamos National Laboratory.* [PDF / Author]
Lucchini J.F., H. Khaing, and D.T. Reed. 2010b. Uranium (VI) Solubility in Carbonate-free ERDA-6 Brine. Scientific Basis for Nuclear Waste Management XXXIV, edited by K.L. Smith, S. Kroeker, B. Uberuaga, K.R. Whittle, Material Research Society Symposium Proceedings, vol.1265: 21-26.* [Author]
Lucchini, J.-F., M.K. Richmann, and M. Borkowski. 2013a. Uranium (VI) Solubility in WIPP Brine. LCO-ACP-14, LANL\ACRSP Report. LA-UR 13-20786. Los Alamos, NM: Los Alamos National Laboratory.* [PDF / Author]
Lucchini, J.-F., M. Borkowski, M.K. Richmann, and D.T. Reed. 2013b. "Uranium(VI) Solubility in carbonate-free WIPP Brine." Radiochim. Acta, 101, 391-398.* [Author]
Lucchini, J.-F., M. Borkowski, H. Khaing, M.K. Richmann, J. Swanson, K. Simmons, and D.T. Reed. 2013c. WIPP Actinide-Relevant Brine Chemistry. LCO-ACP-15, LANL\ACRSP Report. LA-UR 13-20620. Los Alamos, NM: Los Alamos National Laboratory.* [PDF / Author]
Lucchini, J.-F., M. Borkowski, M.K. Richmann, S. Ballard, and D.T. Reed. 2007. "Solubility of Nd3+ and UO22+ in WIPP Brine as Oxidation-State Invariant Analogs for Plutonium." Journal of Alloys and Compounds, vol. 444/445: 506-11. [Author]
Lynd, L., P. Weimer, W. van Zyl, and I. Pretorius. 2002. "Microbial Cellulose Utilization : Fundamentals and Biotechnology." Microbiology and Molecular Biology Reviews, 66(3): p. 506-577.* [Author]
Magirius, S., W.T. Carnall, and J.I. Kim. 1985. "Radiolytic Oxidation of Am(III) to Am(V) in NaCl Solution." Radiochimical Acta, vol. 38: 29-32. [Author]
Martinot, L., and J. Fuger. 1985. "The Actinides." Standard Potentials in Aqueous Solution (pp. 631-674). A.J. Bard, R. Parsons, and J. Jordan, eds. New York: Dekker. [Author]
Masue-Slowey, Y., B.D. Kocar, S.A.B. Jofré, K.U. Mayer, and S. Fendorf. 2011. "Transport Implications Resulting from Internal Redistribution of Arsenic and Iron within Constructed Soil Aggregates." Environmental Science and Technology, vol. 45(2): 582-588.* [Author]
McCabe, A. 1990. "The Potential Significance of Microbial Activity in Radioactive Waste Disposal." Experientia, 46: p. 779-787.* [Author]
Meinrath, G., and J.I. Kim. 1991. "The Carbonate Complexation of the Am(III) Ion." Radiochimica Acta, vol. 52/53: 29-34. [Author]
Meyer, D., S. Fouchard, E. Simoni, and C. DenAuwer. 2002. "Selective Dissolution of Am in Basic Media in the Presence of Ferricyanide Ions: a Mechanistic and Structural Study on Am(V) and Am(VI) Compounds." Radiochimica Acta, vol. 90: 253-58. [Author]
Moriyama, H., T. Sasaki, T. Kobayashi, and I. Takagi. 2005. "Systemics of Hydrolysis Constants of Tetravalent Actinide Ions." Journal of Nuclear Science and Technology, vol. 42-7: 626-35. [Author]
Mormile, M., M. Biesen, M. Gutierrez, A. Ventosa, J. Pavlovich, T. Onstott, and J. Fredrickson. 2003. "Isolation of Halobacterium Salinarum Retrieved Directly from Halite Brine Inclusions." Env. Microbiol., 5(11): 1094-1102.* [Author]
Morss, L.R., N. Edelstein, and J. Fuger. 2006. The Chemistry of the Actinide and Transactinide Elements. 3rd ed. New York: Springer. [Author]
Moulin, C., B. Amekraza, S. Hubert, and V. Moulin. 2001. "Study of Thorium Hydrolysis Species by Electrospray-Ionization Mass Spectrometry." Analytica Chimica Acta, vol. 441: 269-79. [Author]
Myllykylä, E., and K. Ollila. 2011. Reduction of Uranyl Carbonate and Hydroxide Complexes by Ferrous Ions in Aqueous Solution under Anaerobic Conditions. Abstract PA4-3, 13th International Conference on the Chemistry and Migration Behaviour of Actinides and Fission Products in the Geosphere Migration, Beijing, China, September 18-23, 2011.* [Author]
Nakata, K., S. Nagasaki, S. Tanaka, Y. Sakamoto, T. Tanaka, and H. Ogawa. 2004. "Reduction Rate of Neptunium(V) in Heterogeneous Solution with Magnetite." Radiochimica Acta, vol. 92: 145-49. [Author]
National Institute of Standards and Technology (NIST). 2004. Critical Stability Constants.Standard Reference Database 46 (Version 8.0). [Author]
Neck, V., and J.I. Kim. 2001. "Solubility and Hydrolysis of Tetravalent Actinides." Radiochimica Acta, vol. 89: 1-16. [Author]
Neck, V., J.I. Kim, and B. Kanellakopulos. 1992. "Solubility and Hydrolysis Behavior of Neptunium (V)." Radiochimica Acta, vol. 56:, 25-30. [Author]
Neck, V., M. Altmaier, and Th. Fanghänel. 2006. "Ion Interaction (SIT) Coefficients for the Th4+ Ion and Trace Activity Coefficients in NaClO4, NaNO3 and NaCl Solution Determined by Solvent Extraction with TBP." Radiochimica Acta, vol. 94: 501-07. [Author]
Neck, V., M. Altmaier, R. Müller, A. Bauer, Th. Fanghänel, and J.I. Kim. 2003. "Solubility of Crystalline Thorium Dioxide." Radiochimica Acta, vol. 91: 253-62. [Author]
Neck, V., M. Altmaier, T. Rabung, J. Lützenkirchen, and Th. Fänghanel. 2009. "Thermodynamics of Trivalent Actinides and Neodymium in NaCl, MgCl2, and CaCl2 Solutions: Solubility, Hydrolysis and Ternary Ca-M(III)-OH Complexes." Pure and Applied Chemistry, vol. 81(9), 1555-1568. [Author]
Neck, V., R. Müller., M. Bouby, M. Altmaier, J. Rothe, M.A. Denecke, and J.I. Kim. 2002. "Solubility of Amorphous Th(IV) Hydroxide: Application of LIBD to Determine the Solubility Product and EXAFS for Aqueous Speciation." Radiochimica Acta, vol. 90: 485-94. [Author]
Neck, V., W. Runde, and J.I. Kim. 1995. "Solid-Liquid Equilibria of Np(V) in Carbonate Solutions at Different Ionic Strengths: II." Journal of Alloys and Compounds, vol. 225: 295-302. [Author]
Nemer, M., Y. Xiong, A.E. Ismail, and J. Jang. 2011. "Solubility of Fe2(OH)3Cl (pure-iron end-member of hibbingite) in NaCl and Na2SO4 brines." Chemical Geology, v. 280, no. 1-2, 26-32.* [Author]
Neretnieks, I. 1982. The Movement of a Redox Front Downstream From a Repository for Nuclear Waste. KBS Report TR 82-16. Stockholm: Svensk Kärnbränsleforsorjning AB. [Author]
Neu, M.P., C.E. Ruggiero, and A.J. Francis. 2002. Bioinorganic Chemistry of Plutonium and Interactions of Plutonium with Microorganisms in Plants. Advances in Plutonium Chemistry (pp. 1967-2000). La Grange Park, IL: American Nuclear Society. [Author]
Nitsche, H., K. Roberts, K. Becraft, T. Prussin, D. Keeney, S. Carpenter, and D. Hobart. 1995. Solubility and Speciation Results from Over- and Under-saturation Experiments on Neptunium, Plutonium and Americium in water from Yucca Mountain Region Well UE-25p#1. Report LA-13017-MS. Los Alamos, NM: Los Alamos National Laboratory. [Author]
Nitsche, H., K. Roberts, R. Xi, T. Prussin, K. Becraft, I. Al Mahamid, H.B. Silber, S.A. Carpenter, R.C. Gatti, and C.F. Novak. 1994. "Long-Term Plutonium Solubility and Speciation Studies in a Synthetic Brine." Radiochimica Acta, vol. 66/67: 3. [Author]
Nitsche, H., K. Roberts, R.C. Gatti, T. Prussin, K. Becraft, S.C. Leung, S.A. Carpenter, and C.F. Novak. 1992. Plutonium Solubility and Speciation Studies in a Simulant of Air Intake Shaft Water from the Culebra Dolomite at the Waste Isolation Pilot Plant. SAND92-0659. WPO 23480. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Norton, C., and W. Grant. 1988. "Survival of Halobacteria Within Fluid Inclusions in Salt Crystals." J. General Microbiology, 134: 1365-1373.* [Author]
O'Loughlin, E.J., S.D. Kelly, R.E. Cook, R. Csencsits, and K.M. Kemner. 2003. "Reduction of Uranium (VI) by Mixed Iron (II)/ Iron (III) Hydroxide (Green Rust): Formation of UO2 Nanoparticles." Environmental Science and Technology, vol. 37: 721-27. [Author]
Okajima, S., and D.T. Reed. 1993. "Initial Hydrolysis of Pu(VI)." Radiochimica Acta, vol. 60: 173-84. [Author]
Okamoto, Y., Y. Mochizuki, and S. Tsushim. 2003. "Theoretical Study of Hydrolysis Reactions of Tetravalent Thorium Ion." Chemical Physics Letters, vol. 373: 213-17. [Author]
Oren, A. 1999. "Bioenergetic Aspects of Halphilism." Microbiology and Molecular Biology Reviews, 63(2): 334-348.* [Author]
Oren, A. 2006. Life at high salt concentrations. In: Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, Dworkin M, editors. The Prokaryotes. New York: Springer. pp. 263-282.* [Author]
Oren, A. 2008. "Microbial life at high salt concentrations: phylogenetic and metabolic diversity." Saline Systems, vol. 4:2. DOI: 10.1186/1746-1448-4-2.* [Author]
Oren, A. 2011. "Thermodynamic limits to microbial life at high salt concentrations." Environmental Microbiology, vol. 13: 1908-1923.* [Author]
Orlandini, J.A., W.R. Penrose, and D.M. Nelson. 1986. "Pu(V) as the Stable form of Oxidized Plutonium in Natural Waters." Marine Chemistry, vol. 18: 49-57. [Author]
Östhols, E., J. Bruno, and I. Grenthe. 1994. "On the Influence of Carbonate on Mineral Dissolution: III: The Solubility of Microcrystalline ThO2 in CO2-H2O Media." Geochimica et. Cosmochimica Acta, vol. 58: 613-623. [Author]
Oversby, V.M. 2000. Plutonium Chemistry under Conditions Relevant for WIPP Performance Assessment: Review of Experimental Results and Recommendations for Future Work (September). EEG-77. Albuquerque, NM: Environmental Evaluation Group. [Author]
Papenguth, H.W. 1996a. Letter to Christine T. Stockman (Subject: Parameter Record Package for Colloidal Actinide Source Term Parameters, Attachment A: Rationale for Definition of Parameter Values for Microbes). 7 May 1996. ERMS 235856. Carlsbad, NM: Sandia National Laboratories. [Author]
Papenguth, H.W. 1996b. Letter to Christine T. Stockman (Subject: Parameter Record Package for Colloidal Actinide Source Term Parameters, Attachment A: Rationale for Definition of Parameter Values for Humic Substances). 7 May 1996. ERMS 235855. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Papenguth, H.W. 1996c. Letter to Christine T. Stockman (Subject: Parameter Record Package for Colloidal Actinide Source Term Parameters, Attachment A: Rationale for Definition of Parameter Values for Mineral Fragment Type Colloids). 7 May 1996. ERMS 235850. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Papenguth, H.W., and Y.K. Behl. 1996. Test Plan for Evaluation of Colloid-Facilitated Actinide Transport at the Waste Isolation Pilot Plant (16 January). TP 96-01. ERMS 417319. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Pashalidis, I., J.I. Kim, Ch. Lierse, and J.C. Sullivan. 1993. "The Chemistry of Pu in Concentrated Aqueous NaCl Solution: Effects of Alpha Self-Radiolysis and the Interaction Between Hypochlorite and Dioxoplutonium (VI)." Radiochimica Acta, vol. 60: 99. [Author]
Pazukhin, E.M., and S.M. Kochergin. 1989. "Stability Constants of Hydrolyzed Forms of Americium(III) and Solubility Product of its Hydroxide." Soviet Radiochemistry, vol. 31: 430-36. [Author]
Pedersen, K. 2002. Microbial processes in the disposal of high level radioactive waste 500m underground in Fennoscandian shield rocks. In: Keith-Roach MJ, Livens, FR, editors. Interactions of Microorganisms with Radionuclides. London: Elsevier Science Ltd. pp. 279-311.* [Author]
Pitzer, K.S. 1973. "Thermodynamics of Electrolytes. I. Theoretical Basis and General Equations." The Journal of Physical Chemistry, vol. 77(2): 268-277.* [Author]
Popielak, R.S., R.L. Beauheim, S.R. Black, W.E. Coons, C.T. Ellingson, and R.L. Olsen. 1983. Brine Reservoirs in the Castile Formation, Waste Isolation Pilot Plant Project, Southeastern New Mexico. TME 3153. Carlsbad, NM: U.S. Department of Energy. [PDF / Author]
Porter, D., A.N. Roychoudhury, and D. Cowan. 2007. "Dissimilatory Sulfate Reduction in Hypersaline Coastal Pans: Activity Across a Salinity Gradient." Geochimica et Cosmochimica Acta, 71: 5102-5116.* [Author]
Powers, D.W., S.J. Lambert, S.E. Shaffer, I.R. Hill, and W.D. Weart, eds. 1978. Geological Characterization Report, Waste Isolation Pilot Plant (WIPP) Site, Southeastern New Mexico. SAND78-1596. 2 vols. ERMS 205448. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Pryke, D.C., and J.H. Rees. 1986. "Understanding the Behaviour of the Actinides Under Disposal Conditions: A Comparison between Calculated and Experimental Solubilities." Radiochimica Acta, vol. 40: 27-32. [Author]
Qui, S.R., C. Amrhein, M.L. Hunt, R. Pfeffer, B. Yakshinskiy, L. Zhang, T.E. Madey, and J.A. Yarmoff. 2001. "Characterization of Uranium Oxide Thin Films Grown from Solution onto Fe Surfaces." Applied Surface Science, vol. 181: 211-34. [Author]
Rabindra, N.R., K.M. Vogel, C.E. Good, W.B. Davis, L.N. Roy, D.A. Johnson, A.R. Felmy, and K.S. Pitzer. 1992. "Activity Coefficients in Electrolyte Mixtures: HCl + ThCl4 + H2O for 5-55 °C." Journal of Physical Chemistry, vol. 96: 11,065-072. [Author]
Rai, D., A.R. Felmy, and J.L. Ryan. 1990. "Uranium (VI) Hydrolysis Constants and Solubility Products of UO2.xH2O (am)." Inorganic Chemistry, vol. 29: 260-64. [Author]
Rai, D., A.R. Felmy, and R.W. Fulton. 1995. "Nd3+ and Am3+ Ion Interaction with Sulfate Ion and Their Influence on NdPO4(c) Solubility." Journal of Solution Chemistry, vol. 24: 879-95. [Author]
Rai, D., A.R. Felmy, N.J. Hess, and D.A. Moore. 1998. "A Thermodynamic Model for the Solubility of UO2(am) in the Aqueous K+-Na+-HCO3--CO32--OH--H2O System." Radiochimica Acta, vol. 82: 17-25. [Author]
Rai, D., A.R. Felmy, S.M. Sterner, D.A. Moore, M.J. Mason, and C.F. Novak. 1997. "The Solubility of Th(IV) and U(IV) Hydrous Oxides in Concentrated NaCl and MgCl2 Solutions." Radiochimica Acta, vol. 79: 239-47. [Author]
Rai, D., and J.L. Ryan. 1982. "Crystallinity and Solubility of Pu(IV) Oxide and Hydrous Oxide in Aged Aqueous Suspensions." Radiochimica Acta, vol. 30: 213-16. [Author]
Rai, D., and J.L. Ryan. 1985. "Neptunium (IV) Hydrous Oxide Solubility under Reducing and Carbonate Conditions." Inorganic Chemistry, vol. 24: 247-51. [Author]
Rai, D., D.A. Moore, C.S. Oakes, and M. Yui. 2000. "Thermodynamic Model for the Solubility of Thorium Dioxide in the Na+-Cl--OH--H2O System at 23 °C and 90°C." Radiochimica Acta, vol. 88: 297-306. [Author]
Rai, D., J.L. Ryan, D.A. Moore, and R.G. Strickert. 1983. "Am(III) Hydrolysis Constants and Solubility of Am(III) Hydroxide." Radiochimica Acta, vol. 33: 201-06. [Author]
Rai, D., R.G. Strickert, and G.L. McVay. 1982. "Neptunium Concentrations in Solutions Contacting Actinide-Doped Glass." Nuclear Technology, vol. 58: 69-76. [Author]
Rao, L., D. Rai, A.R. Felmy, R.W. Fulton, and C.F. Novak. 1996. "Solubility of NaNd(CO3)2·6H2O(c) in Concentrated NaCO3 and NaHCO3 Solutions." Radiochimica Acta, vol. 75: 141-47. [Author]
Reed, D.T., and D.R. Wygmans. 1997. Actinide Stability/Solubility in Simulated WIPP Brines (March 21). WPO44625. Argonne, IL: Argonne National Laboratory, Actinide Speciation and Chemistry Group, Chemical Technology Group. [Author]
Reed, D.T., J.-F. Lucchini, M. Borkowski, and M.K. Richmann. 2009. Reduction of Higher Valent Plutonium by Iron under Waste Isolation Pilot Plant (WIPP)- Relevant Conditions: Data Summary and Recommendations. LCO-ACP-09, LANL\ACRSP Report. Los Alamos, NM: Los Alamos National Laboratory. [PDF / Author]
Reed, D.T., J.-F. Lucchini, S.B. Aase, and A.J. Kropf. 2006. "Reduction of Plutonium (VI) in Brine under Subsurface Conditions." Radiochimica Acta, vol. 94: 591-97. [Author]
Reed, D.T., J.S. Swanson, J-F. Lucchini, and M.K. Richmann. 2013. Intrinsic, Mineral, and Microbial Colloid Enhancement Parameters for the WIPP Actinide Source Term. Report LCO-ACP-18, LA-UR 13-20858. Carlsbad, NM: Los Alamos National Laboratory.* [Author]
Reed, D.T., R. Deo, and B.E. Rittmann. 2010. Subsurface Interactions of Actinide Species and Microorganisms. In The Chemistry of the Actinide and Transactinide Elements by L.R. Morss, N.M. Edelstein and J. Fuger eds., Chapter 33. Netherlands: Springer Press, 2010.* [Author]
Reed, D.T., S. Okajima, and M.K. Richmann. 1994. "Stability and Speciation of Plutonium(VI) in WIPP Brine." Radiochimica Acta, vol. 66/67: 95-101. [Author]
Reed, D.T., S. Okajima, L.H. Brush, and M.A. Molecke. 1993. Radiolytically Induced Gas Production in Plutonium-Spiked WIPP Brine. Materials Research Society Symposium Proceedings (pp. 431-38). Vol. 294. Warrendale, PA: Materials Research Society. [Author]
Reed, D.T., S.B. Aase, D. Wygmans, and J. E. Banaszak. 1998. "The Reduction of Np(VI) and Pu(VI) by Organic Chelating Agents." Radiochimica Acta, vol. 82: 109-14. [Author]
Reed, D.T., S.E. Pepper, M.K. Richmann, G. Smith, R. Deo, and B.E. Rittmann. 2007. "Subsurface Bio-Mediated Reduction of Higher-Valent Uranium and Plutonium." Journal of Alloys and Compounds, vol. 444/445: 376-82. [Author]
Refait, Ph., and J.-M.R. Génin. 1994. "The Transformation of Chloride-Containing Green Rust One into Sulphated Green Rust Two by Oxidation in Mixed Cl- and SO42- Aqueous Media." Corrosion Science, vol. 36, no. 1: 55-65. [Author]
Richmann, M.K. 2008. Letter report to D. Reed (Subject: Eh/pH Diagrams for Am(III), Th(IV) and Np(V) Based on the FMT Database and Current PA Assumptions). 21 November 2008. Carlsbad, NM: Los Alamos National Laboratory. [PDF / Author]
Rittmann, B.E., J.E. Banaszak, and D.T. Reed. 2002. "Reduction of Np(V) and Precipitation of Np(IV) by an Anaerobic Microbial Consortium." Biodegradation, vol. 13: 329-42. [Author]
Rohban, R., M. Amoozegar, and A. Ventosa. 2009. "Screening and Isolation of Halophilic Bacteria Producing Extracellular Hydrolysis from Howz Soltan Lake, Iran." J. Ind. Microbiol. Biotechnol., 36: 333-340.* [Author]
Roselle, G.T. 2009. Iron and Lead Corrosion in WIPP-Relevant Conditions: Six Month Results. Milestone Report, October 7, 2009. ERMS 546084. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Roselle, G.T. 2010. Iron and Lead Corrosion in WIPP-Relevant Conditions: 12 Month Results. Milestone report, October 14, 2010. ERMS 554383. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Roselle, G.T. 2011a. Iron and Lead Corrosion in WIPP-Relevant Conditions: 18 Month Results. Milestone report, January 5, 2011. ERMS 554715. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Roselle, G.T. 2011b. Iron and Lead Corrosion in WIPP-Relevant Conditions: 24 Month Results. Milestone report, May 3, 2011. ERMS 555246. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Roselle, G.T. 2013. Determination of Corrosion Rates from Iron/Lead Corrosion Experiments to be Used for Gas Generation Calculations, Revision 1. Analysis report, January 23, 2013. ERMS 559077. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Runde, W. 2000. "The Chemical Interactions of Actinides in the Environment." Los Alamos Science, vol. 26: 330. [Author]
Runde, W., and J.I. Kim. 1994. Untersuchungen der Übertragbarkeit von Labordaten natürliche Verhältnisse. Chemisches Verhalten von drei- und fünfwertigem Americium in salinen NaCl-Lösungen. Report RCM-01094, Munich: Institut für Radiochemie, Technische Universität München. (Available from National Technical Information Service, 555 Port Royal Road, Springfield, VA, 22161, 703/487-4650 as DE 95752244.) [Author]
Runde, W., and M. Neu. 2010. Actinide Environmental Chemistry. In The Chemistry of the Actinide and Transactinide Elements by L.R. Morss, N.M. Edelstein and J. Fuger eds., Chapter 32. Netherlands: Springer Press, 2010.* [Author]
Runde, W., S.D. Conradson, D.W. Efurd, N. Lu, D.E. VanPelt, and C.D. Tait. 2002. "Solubility and Sorption of Redox-Sensitive Radionuclides (Np,Pu) in j-13 Water from the Yucca Mountain Site: Comparison Between Experiment and Theory." Applied Geochemistry, vol. 17: 837-53. [Author]
Ryan, J.L., and D. Rai. 1983. "The Solubility of Uranium (IV) Hydrous Oxide in Sodium Hydroxide Solutions under Reducing Conditions." Polyhedron, vol. 2: 947-52. [Author]
Sanchez, A.L., J.W. Murray, and T.H. Sibley. 1985. "The Adsorption of Plutonium IV and V on Geothite." Geochimica Cosmochimica Acta, vol. 49: 2297. [Author]
Sanchez, L.C., and H.R. Trellue. 1996. Memorandum to T. Hicks (Subject: Estimation of Maximum RH-TRU Thermal Heat Load for WIPP). 17 January 1996. WPO 31165. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Schubert, B., T. Lowenstein, and M. Timofeeff. 2009. "Microscipic Identification of Prokaryotes in Modern and Ancient Halite, Saline Valley and Death Valley, California." Astrobiology 9(5): 467-482.* [Author]
Schubert, B., M. Timofeeff, T. Lowenstein, and J. Polle. 2010. "Dunaliella Cells in Fluid Incusions in Halite: Signficance for Long-term Survival of Prokaryotes." Geomicrobiology Journal, 27: 61-75.* [Author]
Schweitzer, G.K., and L.L. Pesterfield. 2010. Chapter 7: The Boron Group. The Aqueous Chemistry of the Elements. (pp. 150-154) Oxford, USA: Oxford University Press.* [Author]
Shannon, R.D. 1976. "Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides." Acta Cryst, vol. A32: 751-67. [Author]
Siekierski, S. 1988. "Comparison of Yttrium, Lanthanides and Actinides in Respect to Unit Cell Volumes of Isostructural Compounds and Thermodynamic Functions of Complex Formation." Journal of Radioanalytical Nuclear Chemistry, vol. 122: 279-84. [Author]
Silva, R.J., G. Bidoglio, M.H. Rand, P.B. Robouch, H. Wanner, and I. Puigdomenech. 1995. Chemical Thermodynamics of Americium. Chemical Thermodynamics Series 2. New York Elsevier. [Author]
Simankova, M.V., and G.A. Zavarzin. 1992. "Anaerobic Degradation of Cellulose from Lake Sivash and Hypersaline Lagoons of the Arabat Spit." Microbiologiya, vol. 61: 288-292.* [Author]
Simankova, M.V., N. Chernych, G. Osipov and G. Zavarzin. 1993. "Halocella celluloytica gen. nov., sp. nov., a New Obligately Anaerobic, Halophilic, Cellulolytic bacterium." System. Appl. Microbio., 16: 385-389.* [Author]
Singer, D.M., S.M. Chatman, E.S. Ilton, K.M. Rosso, J.F. Banfield, and G.A. Waychunas. 2012a. "Identification of Simultaneous U(VI) Sorption Complexes and U(VI) Nanoprecipitates on the Magnetite (111) Surface." Environmental Science & Technology, vol.46: 3811-20.* [Author]
Singer, D.M., S.M. Chatman, E.S. Ilton, K.M. Rosso, J.F. Banfield, and G.A. Waychunas. 2012b. "U(VI) Sorption and Reduction Kinetics on the Magnetite (111) Surface." Environmental Science & Technology, vol.46: 3821-30.* [Author]
Skokan, C.K., M.C. Pfeifer, G.V. Keller, and H.T. Anderses. 1987. Studies of Electrical and Electromagnetic Methods for Characterizing Salt Properties at the WIPP Site, New Mexico. SAND87-7174. Carlsbad NM: Sandia National Laboratories. [PDF / Author]
Snider, A.C. 2003a. Verification of the Definition of Generic Weep Brine and the Development of a Recipe for This Brine. ERMS 527505. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Snider, A.C. 2003b. Hydration of Magnesium Oxide in the Waste Isolation Pilot Plant. Materials Research Society Symposium Proceedings (pp. 665-70). Vol. 757. Warrendale, PA: Materials Research Society. [Author]
Sørensen, K., K. Reháková, E. Zapomĕlová, and A. Oren. 2009. "Distribution of Benthic Phototrophs, Sulfate Reducers, and Methanogens in Two Adjacent Saltern Evaporation Ponds in Eilat, Israel." Aquatic Microbial Ecology, vol. 56: 275-284.* [Author]
Sorokin D., and G. Muyzer. 2010. "Bacterial Dissimilatory MnO2 Reduction at Extremely Haloalkaline Conditions." Extremophiles, 14:41-46.* [Author]
Spinks, J.W.T., and R.J. Woods. 1990. Radiation Sources: The Interaction of Radiation with Matter. Introduction to Radiation Chemistry (pp. 243-313). New York: Wiley. [Author]
Stadler, S., and J.I. Kim. 1988. "Hydrolysis reactions of Am(III) and Am(V)." Radiochim. Acta, 44/45: 39-44. [Author]
Stein, J.S. 2005. Memorandum to L.H. Brush (Subject: Estimate of Volume of Brine in Repository that Leads to a Brine Release). 13 April 2005. ERMS 539372. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Stoelzel, D.M., and D.G. O'Brien. 1996. Analysis Package for the BRAGFLO Direct Release Calculations (Task 4) of the Performance Assessment Analyses Supporting the Compliance Certification Application. AP-029. ERMS 240520. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Suzuki, Y., S.D. Kelly, K.M. Kemner, and J.F. Banfield. 2003. "Microbial populations stimulated for hexavalent uranium reduction in uranium mine sediment." Applied and Environmental Microbiology, 69:1337-46.* [Author]
Swanson, J.S., and K.A. Simmons. 2013. Update on Microbial Characterization of WIPP Groundwaters. Report LCO-ACP-20, LA-UR 13-20623. Carlsbad, NM: Los Alamos National Laboratory.* [Author]
Swanson, J.S., D.M. Norden, H. Khaing, and D.T. Reed. 2013a. "Degradation of Organic Complexing Agents by Halophilic Microorganisms in Brines." Geomicrobiology Journal, 30: 189-98.* [Author]
Swanson, J.S., K.A. Simmons, D.M. Norden, and H. Khaing. 2013b. Biodegradation of Organic Complexing Agents by WIPP-indigenous Halophilic Microorganisms in Brines. Report LCO-ACP-19, LA-UR 13-20616. Carlsbad, NM: Los Alamos National Laboratory.* [Author]
Swanson, J.S., D.T. Reed, D.A. Ams, D.M. Norden, and K.A. Simmons. 2012. Status Report on the Microbial Characterization of Halite and Groundwater Samples from the WIPP. Report LCO-ACP-12, LA-UR 12-22824. Carlsbad, NM: Los Alamos National Laboratory.* [PDF / Author]
Telander, M.R., and R.E. Westerman. 1993. Hydrogen Generation by Metal Corrosion in Simulated Waste Isolation Pilot Plant Environments: Progress Report for the Period November 1989 Through December 1992. SAND92-7347. ERMS 223456. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Telander, M.R., and R.E. Westerman. 1997. Hydrogen Generation by Metal Corrosion in Simulated Waste Isolation Pilot Plant Environments. SAND96-2538. Albuquerque, NM: Sandia National Laboratories. [PDF / Author]
Torrero, M.E., I. Casas, J. de Pablo, M.C.A. Sandino, and B.A. Grambow. 1994. "Comparison Between Unirradiated UO2(s) and Schoepite Solubilities in 1 M NaCl Medium." Radiochimica Acta, vol. 66/67: 29-35. [Author]
Torretto, P., K. Becraft, T. Prussin, K. Roberts, S. Carpenter, D. Hobart, and H. Nitsche. 1995. Solubility and Speciation Results from Oversaturation Experiments on Neptunium, Plutonium and Americium in a Neutral Electrolyte with a Total Carbonate Similar to Water from Yucca Mountain Region Well UE-25p#1. Report LA-13018-MS. Los Alamos, NM: Los Alamos National Laboratory. [PDF / Author]
Trolard, F., J.M.R. Genin, M. Abdelmoula, G. Bourrie, B. Humbert, and A. Herbillon. 1997. "Identification of a Green Rust mineral in a Reductomorphic Soil by Mossbauer and Raman Spectroscopies." Geochimica Cosmochimica Acta, vol. 63: 1107-11. [Author]
U.S. Department of Energy (DOE). 1996. Title 40 CFR Part 191 Compliance Certification Application for the Waste Isolation Pilot Plant (October). 21 vols. DOE/CAO-1996-2184. Carlsbad, NM: Carlsbad Field Office. [Author]
U.S. Department of Energy (DOE). 2004. Title 40 CFR Part 191 Compliance Recertification Application for the Waste Isolation Pilot Plant (March). 10 vols. DOE/WIPP 2004-3231. Carlsbad, NM: Carlsbad Field Office. [Author]
U.S. Department of Energy (DOE). 2009. Title 40 CFR Part 191 Subparts B and C Compliance Recertification Application 2009, Appendix PA, Attachment SOTERM. Carlsbad, NM: Carlsbad Field Office.* [PDF / Author]
U.S. Department of Energy (DOE). 2011. Waste Isolation Pilot Plant Annual Site Environmental Report for 2010. DOE/WIPP-11-2225. Carlsbad, NM: Carlsbad Field Office.* [PDF / Author]
U.S. Environmental Protection Agency (EPA). 1993. "40 CFR Part 191: Environmental Radiation Protection Standards for the Management and Disposal of Spent Nuclear Fuel, High-Level and Transuranic Radioactive Wastes; Final Rule." Federal Register, vol. 58 (December 20, 1993): 66398-416. [PDF / Author]
U.S. Environmental Protection Agency (EPA). 2005. Teleconference with U.S. Department of Energy (DOE), Sandia National Laboratories (SNL), and Los Alamos National Laboratory (LANL) (Subject: Change in U(VI) Solubility Assumption to a Concentration to 1 mM). 2 March 2005. [PDF / Author]
U.S. Environmental Protection Agency (EPA). 2006. Technical Support Document for Section 194.24: Evaluation of the Compliance Recertification Actinide Source Term and Culebra Dolomite Distribution Coefficient Values (March). Washington, DC: Office of Radiation and Indoor Air. [PDF / Author]
Van Loon, L.R., M.A. Glaus, S. Stallone, and A. Laube. 1997. Alkaline Degradation of Cellulose: Estimation of the Concentration of Isosaccharinic Acid in Cement Pore Water. Mat. Res. Soc. Symp. Proc. 506, 1009-1010.* [Author]
Van Luik, A.E., M.J. Apted, W.J. Bailey, J.H. Haberman, J.S. Shade, R.E. Guenther, R.J. Serne, E.R. Gilbert, R. Peters, and R.E. Williford. 1987. Spent Nuclear Fuel as a Waste Form for Geologic Disposal: Assessment and Recommendations on Data and Modeling Needs. PNL-6329. Richland, WA: Pacific Northwest Laboratories. [Author]
Van Soest, G. 2012. Performance Assessment Inventory Report - 2012 (PAIR 2012). INV-PA-12, LANL-CO\Inventory Report, Los Alamos, NM: Los Alamos National Laboratory.* [PDF / Author]
Vandenborre, J., B. Grambov, and A. Abdlouas. 2010. "Discrepancies in Thorium Oxide Solubility Values: Study of Attachment/Detachment Processes at the Solid/Solution Interface." Inorg. Chem., 49, 8736-8748.* [Author]
Villareal, R., J.M. Bergquist, and S.L. Leonard. 2001. The Actinide Source-Term Waste Test Program (STTP): Final Report. 3 vols. LA-UR-01-6822, LA-UR-01-6912, and LA-UR-01-6913. Los Alamos, NM: Los Alamos National Laboratory. [PDF / Author]
Vreeland R.H., A.F. Piselli Jr., S. McDonnough, and S.S. Meyers. 1998. "Distribution and diversity of halophilic bacteria in a subsurface salt formation." Extremophiles 2: 321-331.* [Author]
Wall, N.A., and S.A. Mathews. 2005. "Sustainability of Humic Acids in the Presence of Magnesium Oxide." Applied Geochemistry, vol. 20: 1704-13. [Author]
Walther, C. 2003. "Comparison of Colloid Investigations by Single Particle Analytical Techniques: A Case Study on Thorium-Oxyhydroxides." Colloids and Surfaces A: Physicochemical Engineering Aspects, vol. 217: 81-92. [Author]
Wang Y., and A.J. Francis. 2005. "Evaluation of microbial activity for long-term performance assessments of deep geologic nuclear waste repositories." Journal of Nuclear and Radiochemical Science 6: 43-50.* [Author]
Wang, Y., and L.H. Brush. 1996. Memorandum to M.S. Tierney (Subject: Estimates of Gas-Generation Parameters for the Long-Term WIPP Performance Assessment). 26 January 1996. ERMS 231943. Carlsbad, NM: Sandia National Laboratories. [PDF / Author]
Wang, Z., RC. Moore, A.R. Felmy, M.J. Mason, and R.K. Kukkadapu. 2001. "A study of the corrosion products of mild steel in high ionic strength brines." Waste Management 21:335-341.* [Author]
Warwick, P., N. Evans, and S.Vines. 2006. "Studies on some divalent metal α-isosaccharinic acid complexes." Radiochim. Acta 94, 363-368.* [Author]
Weiner, R. 1996. Technical memorandum to SWCF-A: Records Center (Subject: Documentation Package For: Oxidation State Distribution of Actinides in the Repository). 27 March 1996. ERMS 235194. Albuquerque, NM: Sandia National Laboratories. [Author]
White, A.F., A. Yee, and S. Flexser. 1985. "Surface Oxidation-Reduction Kinetics Associated with Experimental Basalt-Water Reactions at 25 °C." Chemical Geology, vol. 49: 73. [Author]
Wilson D. 2011. "Microbial Diversity of Cellulose Hydrolysis." Current Opinion in Microbiology (Ecology and Industrial Microbiology), 14: 259-263.* [Author]
Wolery, T.J. 1992. EQ3/6, A Software Package for Geochemical Modeling of Aqueous Systems: Package Overview and Installation Guide (Version 7.0). UCRL-MA-110662 PT 1. Livermore, CA: Lawrence Livermore National Laboratory. [Author]
Wolery, T.J. 2008. Analysis Plan for EQ3/6 Analytical Studies. AP-140, Rev. 0., 15 May 2008. ERMS 548930. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Wolery, T.J., and R.L. Jarek. 2003. Software User's Manual: EQ3/6, Version 8.0. Software Document No. 10813-UM-8.0-00. Albuquerque, NM: Sandia National Laboratories.* [PDF / Author]
Wolery, T.J., and S.A. Daveler. 1992. EQ3/6, A Computer Program for Reaction Path Modeling of Aqueous Geochemical Systems: Theoretical Manual, User's Guide, and Related Documentation (Version 7.0). UCRL-MA-110662-Pt. 4. Livermore, CA: Lawrence Livermore National Laboratory. [Author]
Wolery, T.J., Y.-L. Xiong, and J.J. Long. 2010. Verification and Validation Plan/Validation Document for EQ3/6 Version 8.0a for Actinide Chemistry, Document Version 8.10. ERMS 550239. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Xiong, Y.-L. 2011a. E-mail to Jennifer Long (Subject: Release of EQ3/6 Database DATA0.FM1). 9 March 2011. ERMS 555152. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Xiong, Y.-L. 2011b. WIPP Verification and Validation Plan/Validation Document for EQ3/6 Version 8.0a for Actinide Chemistry, Revision 1. Supersedes ERMS 550239. ERMS 555358. Carlsbad, NM: Sandia National Laboratories.* [PDF / Author]
Xiong, Y.-L., and A.S. Lord. 2008. "Experimental Investigations of the Reaction Path in the MgO-CO2-H2O System in Solution with Various Ionic Strengths, and Their Applications to Nuclear Waste Isolation." Applied Geochemistry, vol. 23: 1634-59. [Author]
Yajima, T., Y. Kawamura, and S. Ueta. 1995. Uranium(IV) Solubility and Hydrolysis Constants Under Reduced Conditions. Materials Research Society Symposium Proceedings, (pp. 1137-42). Vol. 353. Warrendale, PA: Materials Research Society. [Author]
Yamamura, T., A. Kitamura, A. Fukui, S. Nishikawa, T. Yamamoto, and H. Moriyama. 1998. "Solubility of U(VI) in Highly Basic Solutions." Radiochimica Acta, vol. 83: 139-146. [Author]
Yamazaki, H., B. Lagerman, V. Symeopoulos, and G.R. Choppin. 1992. "Solubility of Uranyl in Brine." Radioactive Waste Management, vol. 1992: 1607-11. [Author]
Zitomer, D.H., and R.E. Speece. 1993. "Sequential Environments for Enhanced Biotransformation of Aqueous Contaminants." Environmental Science and Technology, vol. 27: 227. [Author]